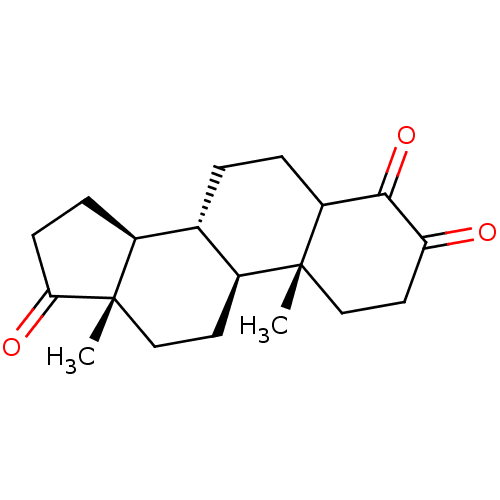
BDBM50240798 (8R,9S,10R,13S,14S)-4-Hydroxy-10,13-dimethyl-1,6,7,8,9,10,11,12,13,14,15,16-dodecahydro-2H-cyclopenta[a]phenanthrene-3,17-dione::4-hyroxyandrostenedione::CHEMBL132530::FORMESTANE
SMILES C[C@]12CC[C@H]3[C@@H](CCC4C(=O)C(=O)CC[C@]34C)[C@@H]1CCC2=O
InChI Key InChIKey=ZMEULISFKSVRLQ-AZXRADLDSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 18 hits for monomerid = 50240798
Found 18 hits for monomerid = 50240798
Affinity DataKi: 27nMAssay Description:Inhibition constant for human placental cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataKi: 43nMAssay Description:Irreversible inhibition of human aromatase extracted from placental microsomesMore data for this Ligand-Target Pair
Affinity DataKi: 50nMAssay Description:Binding affinity was measured on Cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataKi: 54nMAssay Description:Evaluated for its competitive inhibitory activity against Cytochrome P450 19A1 with the use of human placental microsomal preparationMore data for this Ligand-Target Pair
Affinity DataKi: 542nMAssay Description:Binding affinity(KI) for formation of reversible enzyme-ligand complex with human placental aromatase (PL2)More data for this Ligand-Target Pair
Affinity DataIC50: 92nMAssay Description:Inhibition of human placental aromatase assessed as conversion of [1beta-3H]androstenedione to [1beta-3H]estrone after 20 mins by liquid scintillatio...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibition of aromatase in human breast tumorMore data for this Ligand-Target Pair
Affinity DataIC50: 42nMAssay Description:Inhibitory concentration against aromatase protein from human placental microsomes using [1-beta-3H]-androstenedione; Competitive inhibitionMore data for this Ligand-Target Pair
Affinity DataIC50: 5.86E+4nMAssay Description:Inhibition of aromatase (unknown origin) using dibenzylfluorescein as substrate after 30 mins by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 48.6nMAssay Description:Inhibition of human placental aromatase using [3H]-1beta-androstenedione as substrate after 16 hrs by [3H]-water methodMore data for this Ligand-Target Pair
Affinity DataIC50: 42nMAssay Description:Inhibition of aromatase in human placental microsomes using [1beta-3H]androstenedione as substrate after 15 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 370nMAssay Description:Inhibition of cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibition of particulate fractions of human breast cancer derived aromataseMore data for this Ligand-Target Pair
Affinity DataIC50: 370nMAssay Description:Inhibition of cytochrome P450 19A1 aromatase from human placental microsomesMore data for this Ligand-Target Pair
Affinity DataIC50: 800nMAssay Description:Inhibition of aromatase in human placental microsomes assessed as tritiated water release after 15 mins using [1beta, 3H]androstenedione as substrate...More data for this Ligand-Target Pair
Affinity DataIC50: 470nMAssay Description:Inhibition of human aromatase using androstenedione as substrate assessed as estrone formation at 10 uM after 30 mins by LC-MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 410nMAssay Description:Inhibition of 1 uM [1-beta-3H]-androstenedione binding to human placental microsome Cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataIC50: 42nMAssay Description:Inhibition of human placental aromataseChecked by AuthorMore data for this Ligand-Target Pair