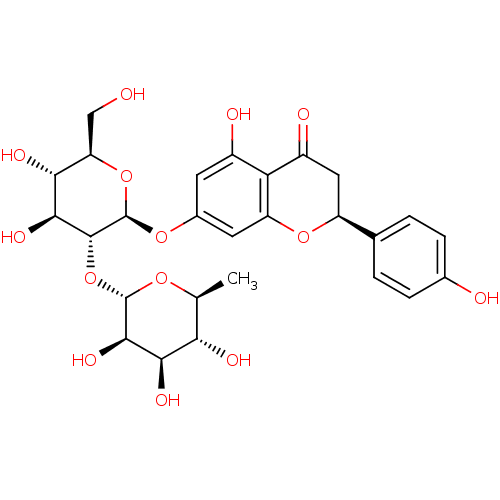
BDBM50241582 (S)-7-((2S,3R,4S,5S,6R)-4,5-dihydroxy-6-(hydroxymethyl)-3-((2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro-2H-pyran-2-yloxy)-tetrahydro-2H-pyran-2-yloxy)-5-hydroxy-2-(4-hydroxyphenyl)chroman-4-one::CHEMBL451532::cid_25075::cid_442428::naringin
SMILES C[C@@H]1O[C@@H](O[C@@H]2[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]2Oc2cc(O)c3C(=O)C[C@H](Oc3c2)c2ccc(O)cc2)[C@H](O)[C@H](O)[C@H]1O
InChI Key InChIKey=DFPMSGMNTNDNHN-ZPHOTFPESA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 13 hits for monomerid = 50241582
Found 13 hits for monomerid = 50241582
TargetG protein-activated inward rectifier potassium channel 4(Homo sapiens (Human))
Vanderbilt University
Curated by ChEMBL
Vanderbilt University
Curated by ChEMBL
Affinity DataEC50: >1.00E+5nMAssay Description:Activation of recombinant human GIRK1/4 expressed in Xenopus oocytes by two-electrode voltage clamp methodMore data for this Ligand-Target Pair
TargetG protein-activated inward rectifier potassium channel 1(Homo sapiens (Human))
Vanderbilt University
Curated by ChEMBL
Vanderbilt University
Curated by ChEMBL
Affinity DataEC50: >1.00E+5nMAssay Description:Activation of recombinant human GIRK1/2 expressed in Xenopus oocytes by two-electrode voltage clamp methodMore data for this Ligand-Target Pair
TargetUbiquitin-conjugating enzyme E2 N(Homo sapiens (Human))
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 8.70E+3nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego CA...More data for this Ligand-Target Pair
TargetUbiquitin-conjugating enzyme E2 N(Homo sapiens (Human))
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 5.77E+3nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego CA...More data for this Ligand-Target Pair
TargetBcl-2-related protein A1(Mus musculus (Mouse))
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: >2.00E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego CA...More data for this Ligand-Target Pair
TargetBcl-2-related protein A1(Mus musculus (Mouse))
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: >2.00E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego CA...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of Homo sapiens (human) recombinant GSK3beta after 30 min by Kinase-Glo assayMore data for this Ligand-Target Pair
TargetUbiquitin-conjugating enzyme E2 N(Homo sapiens (Human))
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 6.66E+3nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego CA...More data for this Ligand-Target Pair
Affinity DataKd: >1.00E+6nMAssay Description:Binding affinity to recombinant heparanase catalytic stie (unknown origin) expressed in Escherichia coli BL21 (DE3) by surface plasmon resonance assa...More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of aromataseMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Korea Research Institute Of Bioscience And Biotechnology
Curated by ChEMBL
Korea Research Institute Of Bioscience And Biotechnology
Curated by ChEMBL
Affinity DataIC50: >6.00E+4nMAssay Description:Inhibition of human recombinant PTP1BMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Korea Research Institute Of Bioscience And Biotechnology
Curated by ChEMBL
Korea Research Institute Of Bioscience And Biotechnology
Curated by ChEMBL
Affinity DataIC50: 9.18E+4nMAssay Description:Inhibition of PTP1B (unknown origin) using pNPP as substrate pretreated for 10 mins followed by substrate addition measured after 20 mins by spectrop...More data for this Ligand-Target Pair
TargetUbiquitin-conjugating enzyme E2 N(Homo sapiens (Human))
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 1.22E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego CA...More data for this Ligand-Target Pair