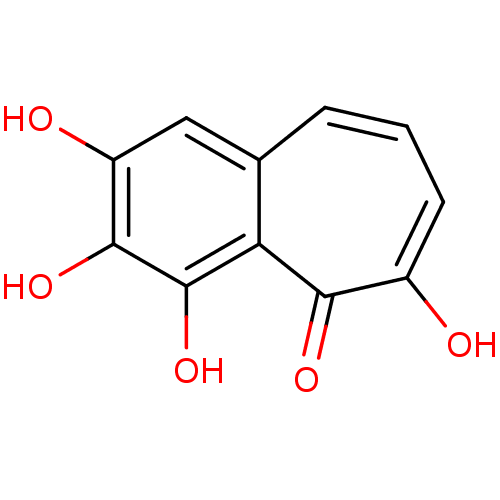
BDBM50088360 2,3,4,6-tetrahydroxy-5H-benzo[7]annulen-5-one::2,3,4,6-tetrahydroxy-5H-benzocycloheptene-5-one::2,3,4,6-tetrahydroxybenzocyclohepten-5-one::CHEMBL66953::Hit compound, 2::Purpurogallin::purpurogalline
SMILES Oc1cc2cccc(O)c(=O)c2c(O)c1O
InChI Key InChIKey=WDGFFVCWBZVLCE-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 50088360
Found 9 hits for monomerid = 50088360
Affinity DataIC50: 1.70E+4nMAssay Description:The inhibitory against activated CDK2-cyclin A2 complex was determined by using the ADP Quest fluorescence assay from (DiscoveRX, Fremont, CA)More data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+4nMAssay Description:Competitive inhibition of human CDK2/cyclinA using PKTPKKAKKL as substrate in presence of ATPMore data for this Ligand-Target Pair
Affinity DataIC50: 2.10E+3nMAssay Description:Inhibition of HIV-1 integrase, under 1 uM for the 3''-preprocessingMore data for this Ligand-Target Pair
Affinity DataIC50: 2.20E+3nMAssay Description:Inhibitory concentration against Bcl-xlMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2(Homo sapiens (Human))
University Of East Anglia
Curated by ChEMBL
University Of East Anglia
Curated by ChEMBL
Affinity DataIC50: 7.30E+3nMAssay Description:Inhibition of human SHIP2 catalytic domain (419 to 832 residues) phosphatase activity assessed as inhibition of Ins(1,3,4,5)P4 production using Ins(1...More data for this Ligand-Target Pair
Affinity DataIC50: 1.29E+4nMAssay Description:Inhibition of human alpha-synuclein filament formation expressed in Escherichia coli BL21(DE3) cells incubated for 72 hrs by thioflavin S based fluor...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2(Homo sapiens (Human))
University Of East Anglia
Curated by ChEMBL
University Of East Anglia
Curated by ChEMBL
Affinity DataIC50: 5.50E+3nMAssay Description:Inhibition of 2-FAM-InsP5 binding to human SHIP2 catalytic domain (419 to 832 residues) assessed as change in polarization by fluorescence polarizati...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2(Homo sapiens (Human))
University Of East Anglia
Curated by ChEMBL
University Of East Anglia
Curated by ChEMBL
Affinity DataIC50: 8.90E+4nMAssay Description:Inhibition of human SHIP2 catalytic domain (419 to 832 residues) phosphatase activity assessed as phosphate release using Ins(1,3,4,5)P4 as substrate...More data for this Ligand-Target Pair
Affinity DataIC50: 6.00E+4nMAssay Description:Inhibition of recombinant human IDO1 expressed in bacterial expression system using L-Tryptophan as substrate after 25 mins in absence of GSH and pre...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)