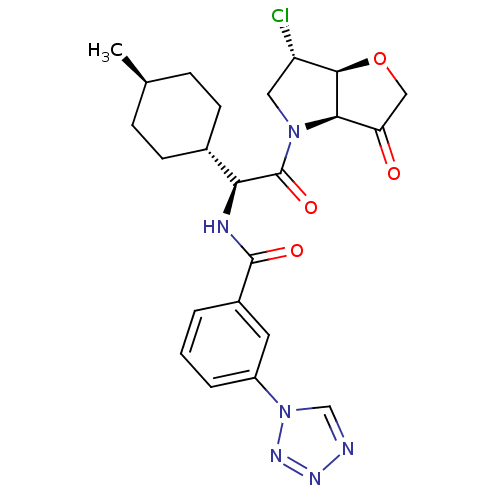
BDBM103361 US8552202, Example 2
SMILES C[C@H]1CC[C@@H](CC1)[C@H](NC(=O)c1cccc(c1)-n1cnnn1)C(=O)N1C[C@H](Cl)[C@H]2OCC(=O)[C@@H]12
InChI Key InChIKey=BNTXXZPUXMSHRP-UEWVETAJSA-N
Data 5 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 103361
Found 5 hits for monomerid = 103361
Affinity DataKi: 2.20nMAssay Description:In vitro inhibition assay using cathepsin.More data for this Ligand-Target Pair
Affinity DataKi: >8.00E+3nMAssay Description:In vitro inhibition assay using cathepsin.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:In vitro inhibition assay using cathepsin.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:In vitro inhibition assay using cathepsin.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:In vitro inhibition assay using cathepsin.More data for this Ligand-Target Pair