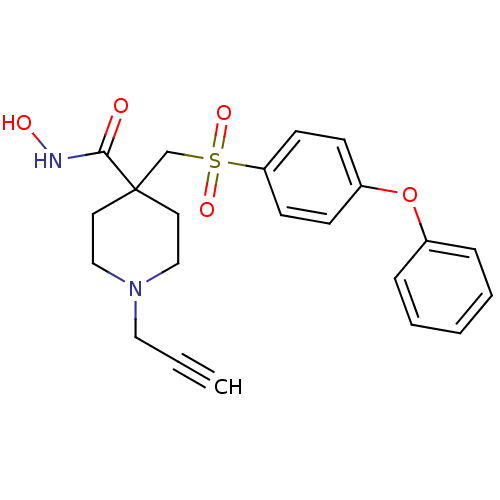
BDBM11868 CHEMBL256157::N-Hydroxy-4-{[(4-phenoxyphenyl)sulfonyl]methyl}-1-prop-2-ynylpiperidine-4-carboxamide Hydrochloride::N-hydroxy-4-{[(4-phenoxybenzene)sulfonyl]methyl}-1-(prop-2-yn-1-yl)piperidine-4-carboxamide hydrochloride::beta-sulfone 7e
SMILES ONC(=O)C1(CS(=O)(=O)c2ccc(Oc3ccccc3)cc2)CCN(CC#C)CC1
InChI Key InChIKey=PRJCFROAJDAXAD-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 10 hits for monomerid = 11868
Found 10 hits for monomerid = 11868
Affinity DataKi: 0.25nMAssay Description:Inhibitors were assayed against purified hMMP-1, hMMP-2, hMMP-3, hMMP-8, hMMP-9, and hMMP-13 using an enzyme assay based on cleavage of the quenched ...More data for this Ligand-Target Pair
Affinity DataKi: 0.550nMAssay Description:Inhibitors were assayed against purified hMMP-1, hMMP-2, hMMP-3, hMMP-8, hMMP-9, and hMMP-13 using an enzyme assay based on cleavage of the quenched ...More data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:Inhibitors were assayed against purified hMMP-1, hMMP-2, hMMP-3, hMMP-8, hMMP-9, and hMMP-13 using an enzyme assay based on cleavage of the quenched ...More data for this Ligand-Target Pair
Affinity DataKi: 4.5nMAssay Description:Inhibitors were assayed against purified hMMP-1, hMMP-2, hMMP-3, hMMP-8, hMMP-9, and hMMP-13 using an enzyme assay based on cleavage of the quenched ...More data for this Ligand-Target Pair
Affinity DataKi: 35nMAssay Description:Inhibitors were assayed against purified hMMP-1, hMMP-2, hMMP-3, hMMP-8, hMMP-9, and hMMP-13 using an enzyme assay based on cleavage of the quenched ...More data for this Ligand-Target Pair
Affinity DataKi: 417nM ΔG°: -8.70kcal/molepH: 7.5 T: 2°CAssay Description:Inhibitors were assayed against purified hMMP-1, hMMP-2, hMMP-3, hMMP-8, hMMP-9, and hMMP-13 using an enzyme assay based on cleavage of the quenched ...More data for this Ligand-Target Pair
Affinity DataIC50: 8.66E+3nMAssay Description:Inhibition of MMP1More data for this Ligand-Target Pair