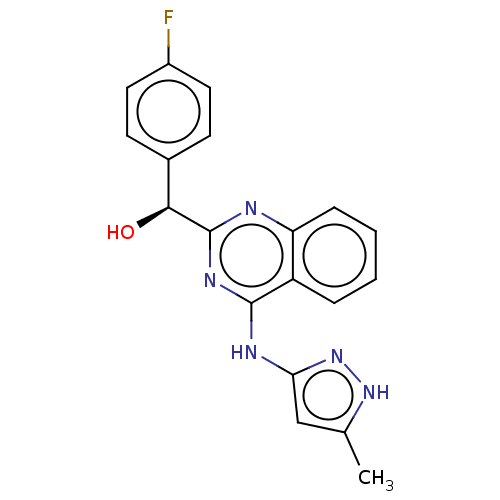
BDBM214689 US9295672, (S)-(4-fluorophenyl)(4-((5-methyl-1H-pyrazol-3-yl)amino)quinazolin-2-yl)methanol
SMILES Cc1cc(Nc2nc(nc3ccccc23)[C@@H](O)c2ccc(F)cc2)n[nH]1
InChI Key InChIKey=DCRWIATZWHLIPN-KRWDZBQOSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 16 hits for monomerid = 214689
Found 16 hits for monomerid = 214689
Affinity DataKd: 2.50E+3nMT: 2°CAssay Description:For the binding assays, streptavidin-coated magnetic beads were treated with biotinylated affinity ligands for 30 min at room temperature to generate...More data for this Ligand-Target Pair
Affinity DataKd: 5nMAssay Description:Inhibition of JAK3 (unknown origin) assessed as dissociate constantMore data for this Ligand-Target Pair
Affinity DataKd: 5.00E+3nMT: 2°CAssay Description:For the binding assays, streptavidin-coated magnetic beads were treated with biotinylated affinity ligands for 30 min at room temperature to generate...More data for this Ligand-Target Pair
Affinity DataKd: 1.60E+3nMT: 2°CAssay Description:For the binding assays, streptavidin-coated magnetic beads were treated with biotinylated affinity ligands for 30 min at room temperature to generate...More data for this Ligand-Target Pair
Affinity DataIC50: 1.55nMpH: 7.4 T: 2°CAssay Description:.(R)- and (S)-(4-Fluorophenyl)(4-((5-methyl-1H-pyrazol-3-yl)amino)quinazolin-2-yl)methanol was tested in a radioligand binding assay, according to th...More data for this Ligand-Target Pair
Affinity DataKd: 1.42nMpH: 7.4 T: 2°CAssay Description:.(R)- and (S)-(4-Fluorophenyl)(4-((5-methyl-1H-pyrazol-3-yl)amino)quinazolin-2-yl)methanol was tested in a radioligand binding assay, according to th...More data for this Ligand-Target Pair
Affinity DataIC50: <310nMT: 2°CAssay Description:The ability of the R and S enantiomers of (4-fluorophenyl)(4-((5-methyl-1H-pyrazol-3-yl)amino)quinazolin-2-yl)methanol to inhibit the common drug met...More data for this Ligand-Target Pair
Affinity DataIC50: >4.00E+4nMT: 2°CAssay Description:The ability of the R and S enantiomers of (4-fluorophenyl)(4-((5-methyl-1H-pyrazol-3-yl)amino)quinazolin-2-yl)methanol to inhibit the common drug met...More data for this Ligand-Target Pair
Affinity DataIC50: 2.27E+4nMT: 2°CAssay Description:The ability of the R and S enantiomers of (4-fluorophenyl)(4-((5-methyl-1H-pyrazol-3-yl)amino)quinazolin-2-yl)methanol to inhibit the common drug met...More data for this Ligand-Target Pair
Affinity DataIC50: 2.46E+4nMT: 2°CAssay Description:The ability of the R and S enantiomers of (4-fluorophenyl)(4-((5-methyl-1H-pyrazol-3-yl)amino)quinazolin-2-yl)methanol to inhibit the common drug met...More data for this Ligand-Target Pair
Affinity DataIC50: 1.59E+4nMT: 2°CAssay Description:The ability of the R and S enantiomers of (4-fluorophenyl)(4-((5-methyl-1H-pyrazol-3-yl)amino)quinazolin-2-yl)methanol to inhibit the common drug met...More data for this Ligand-Target Pair
Affinity DataIC50: >4.00E+4nMT: 2°CAssay Description:The ability of the R and S enantiomers of (4-fluorophenyl)(4-((5-methyl-1H-pyrazol-3-yl)amino)quinazolin-2-yl)methanol to inhibit the common drug met...More data for this Ligand-Target Pair
Affinity DataIC50: 1.05E+4nMT: 2°CAssay Description:The ability of the R and S enantiomers of (4-fluorophenyl)(4-((5-methyl-1H-pyrazol-3-yl)amino)quinazolin-2-yl)methanol to inhibit the common drug met...More data for this Ligand-Target Pair
Affinity DataKd: 0.180nMAssay Description:Inhibition of JAK2 (unknown origin) assessed as dissociate constantMore data for this Ligand-Target Pair
Affinity DataKd: 2.5nMAssay Description:Inhibition of JAK1 (unknown origin) assessed as dissociate constantMore data for this Ligand-Target Pair
Affinity DataKd: 180nMT: 2°CAssay Description:For the binding assays, streptavidin-coated magnetic beads were treated with biotinylated affinity ligands for 30 min at room temperature to generate...More data for this Ligand-Target Pair