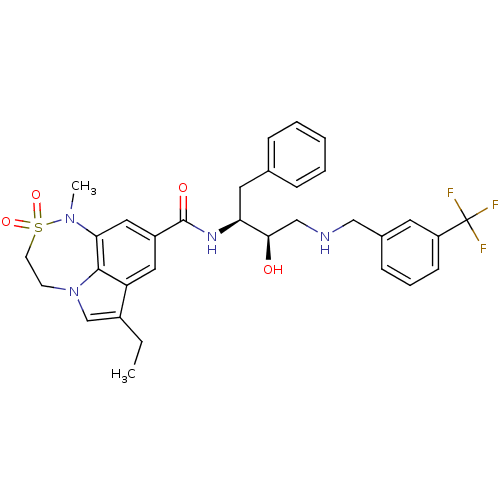
BDBM26503 3-ethyl-N-[(2S,3R)-3-hydroxy-1-phenyl-4-({[3-(trifluoromethyl)phenyl]methyl}amino)butan-2-yl]-9-methyl-10,10-dioxo-10-thia-1,9-diazatricyclo[6.4.1.0^{4,13}]trideca-2,4(13),5,7-tetraene-6-carboxamide::BMCL193669 Compound 9::tricyclic hydroxyethylamine (HEA) derivative, 8a
SMILES CCc1cn2CCS(=O)(=O)N(C)c3cc(cc1c23)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNCc1cccc(c1)C(F)(F)F
InChI Key InChIKey=MSHYGGHZTGSTOG-LMSSTIIKSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 26503
Found 9 hits for monomerid = 26503
Affinity DataIC50: 2nMpH: 4.5 T: 2°CAssay Description:Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e...More data for this Ligand-Target Pair
Affinity DataIC50: 59nMpH: 4.5 T: 2°CAssay Description:Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e...More data for this Ligand-Target Pair
Affinity DataIC50: 1.26E+3nMAssay Description:Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMpH: 4.5 T: 2°CAssay Description:Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e...More data for this Ligand-Target Pair
Affinity DataIC50: 1.26E+3nMAssay Description:Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e...More data for this Ligand-Target Pair
Affinity DataIC50: 1.26E+3nMAssay Description:Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMpH: 4.5 T: 2°CAssay Description:Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e...More data for this Ligand-Target Pair
Affinity DataIC50: 59nMAssay Description:Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e...More data for this Ligand-Target Pair
Affinity DataIC50: 59nMAssay Description:Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)