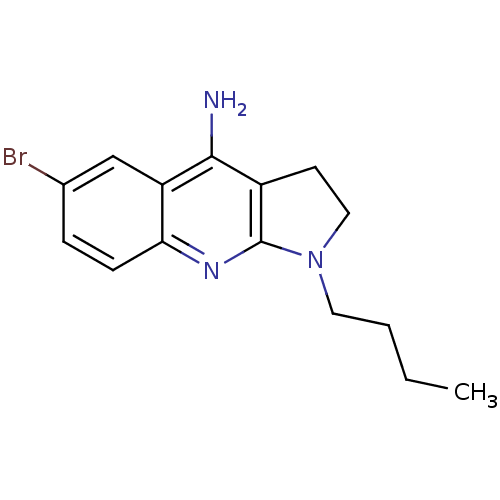
BDBM39664 (6-bromo-1-butyl-2,3-dihydropyrrolo[2,3-b]quinolin-4-yl)amine;hydrochloride::6-bromanyl-1-butyl-2,3-dihydropyrrolo[2,3-b]quinolin-4-amine;hydrochloride::6-bromo-1-butyl-2,3-dihydro-1H-pyrrolo[2,3-b]quinolin-4-amine hydrochloride::6-bromo-1-butyl-2,3-dihydropyrrolo[2,3-b]quinolin-4-amine;hydrochloride::MLS000532161::SMR000137102::cid_2831789
SMILES CCCCN1CCc2c1nc1ccc(Br)cc1c2N
InChI Key InChIKey=XHNLICSHYXRRRU-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 39664
Found 5 hits for monomerid = 39664
TargetAlkaline phosphatase, germ cell type(Homo sapiens (Human))
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 9.99E+5nMAssay Description:Sanford-Burnham Center for Chemical Genomics (SBCCG) Sanford-Burnham Medical Research Institute (SBMRI, San Diego, CA) NIH Molecular Libraries Screen...More data for this Ligand-Target Pair
TargetAlkaline phosphatase, germ cell type(Homo sapiens (Human))
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataEC50: 7.72E+4nMAssay Description:Sanford-Burnham Center for Chemical Genomics (SBCCG) Sanford-Burnham Medical Research Institute (SBMRI, San Diego, CA) NIH Molecular Libraries Screen...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Nanjing University Of Chinese Medicine
Curated by ChEMBL
Nanjing University Of Chinese Medicine
Curated by ChEMBL
Affinity DataIC50: 300nMAssay Description:Inhibition of Electrophorus electricus AChE pre-incubated for 5 mins before acetylthiocholine iodide substrate addition by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 8.92E+3nMAssay Description:Inhibition of equine serum BChE pre-incubated for 5 mins before butyrylthiocholineiodide substrate addition by Ellman's methodMore data for this Ligand-Target Pair
TargetXylosyl- and glucuronyltransferase LARGE1(Homo sapiens (Human))
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay