BDBM50007788 CHEMBL3233809::med.21724, Compound 167
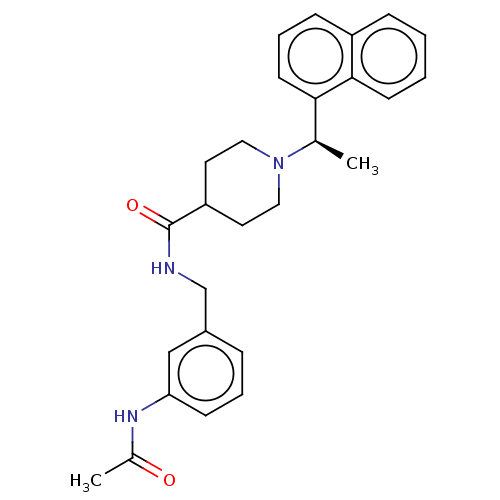
SMILES C[C@@H](N1CCC(CC1)C(=O)NCc1cccc(NC(C)=O)c1)c1cccc2ccccc12
InChI Key InChIKey=YQRGVYYHRJBSOR-LJQANCHMSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 10 hits for monomerid = 50007788
Found 10 hits for monomerid = 50007788
TargetUbiquitin carboxyl-terminal hydrolase 2(Homo sapiens (Human))
Purdue University
Curated by ChEMBL
Purdue University
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of human USP2 using Ub-rhodamine 110 as substrateMore data for this Ligand-Target Pair
TargetUbiquitin carboxyl-terminal hydrolase 7(Homo sapiens (Human))
Purdue University
Curated by ChEMBL
Purdue University
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of human USP7 using Ub-rhodamine 110 as substrateMore data for this Ligand-Target Pair
TargetUbiquitin carboxyl-terminal hydrolase 8(Homo sapiens (Human))
Purdue University
Curated by ChEMBL
Purdue University
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of human USP8 using Ub-rhodamine 110 as substrateMore data for this Ligand-Target Pair
TargetUbiquitin carboxyl-terminal hydrolase 20(Homo sapiens (Human))
Purdue University
Curated by ChEMBL
Purdue University
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of human USP20 using Ub-rhodamine 110 as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 2.69E+3nMAssay Description:Inhibition of full-length wild-type SARS-CoV-2 papain-like protease (1564 to 1878 residues) expressed in Escherichia coli BL21 (DE3) using HCC-RLRGG-...More data for this Ligand-Target Pair
Affinity DataIC50: 4.60E+4nMAssay Description:Inhibition of human coronavirus NL63 PLP2 (amino acids 1565 to 1894) expressed in Escherichia coli BL21 (DE3) cells assessed as reduction of AMC rele...More data for this Ligand-Target Pair
Affinity DataIC50: 390nMAssay Description:This is a review article.More data for this Ligand-Target Pair
Affinity DataIC50: 2.69E+3nMAssay Description:Inhibition of full-length SARS-CoV-2 PLpro expressed in Escherichia coli BL21(DE3) using HCC-RLRGG-NH(CH2)4NH-DABCYL probe as substrate incubated for...More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of full-length SARS-CoV-2 PLpro expressed in Escherichia coli BL21(DE3) using Ub-Rho as substrate incubated for 5 mins by enzymatic assayMore data for this Ligand-Target Pair
TargetUbiquitin carboxyl-terminal hydrolase 21(Homo sapiens (Human))
Purdue University
Curated by ChEMBL
Purdue University
Curated by ChEMBL
Affinity DataIC50: >3.10E+4nMAssay Description:Inhibition of human USP21 using Ub-rhodamine 110 as substrateMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)