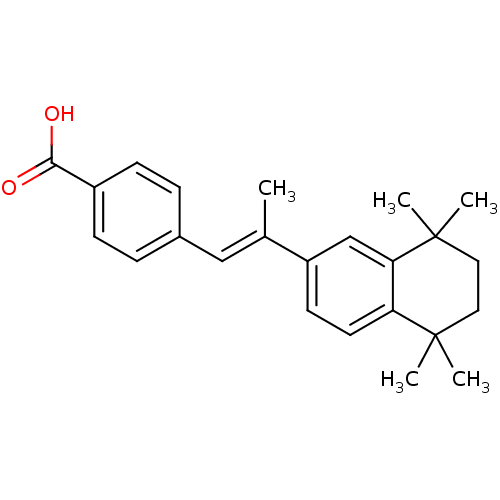
BDBM50032219 (E)-4-(2-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthalen-2-yl)prop-1-enyl)benzoic acid::4-(2-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthalen-2-yl)prop-1-enyl)benzoic acid::4-[(1E)-2-(5,5,8,8-TETRAMETHYL-5,6,7,8-TETRAHYDRONAPHTHALEN-2-YL)PROP-1-ENYL]BENZOIC ACID::4-[(E)-2-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-naphthalen-2-yl)-propenyl]-benzoic acid::4-[(E)-2-(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl)-1-propenyl]benzoic acid::4-[2-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-naphthalen-2-yl)-propenyl]-benzoic acid::CHEMBL275311::US9963439, TTNPB
SMILES C\C(=C/c1ccc(cc1)C(O)=O)c1ccc2c(c1)C(C)(C)CCC2(C)C
InChI Key InChIKey=FOIVPCKZDPCJJY-JQIJEIRASA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 45 hits for monomerid = 50032219
Found 45 hits for monomerid = 50032219
Affinity DataKi: 0.5nMAssay Description:Binding affinity towards cRARbeta2 receptor by displacing 1.1 nM 3[H]-9-cis-RAMore data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Binding affinity towards cRARbeta2 receptor by displacing 0.82 nM 3[H]-all-trans-RAMore data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Binding affinity to retinoic acid receptor beta using [3H]-CD 367 as radioligandMore data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Binding affinity to retinoic acid receptor (RAR) gamma using [3H]-CD 367 as radioligandMore data for this Ligand-Target Pair
Affinity DataKi: 21nMAssay Description:Binding affinity to retinoic acid receptor alpha using [3H]-CD 367 as radioligandMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+3nMAssay Description:Binding affinity against retinoic Acid X beta receptor using [3H]- -9-cis-Retinoic Acid in competitive binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+3nMAssay Description:Binding affinity against retinoic Acid X alpha receptor using [3H]- -9-cis-Retinoic Acid in competitive binding assayMore data for this Ligand-Target Pair
Affinity DataEC50: 2nMAssay Description:Binding affinity against retinoic Acid gamma receptors cotransfected into CV-1 cellsMore data for this Ligand-Target Pair
Affinity DataEC50: >1.00E+4nMAssay Description:Binding affinity against retinoic Acid X gamma receptors cotransfected into CV-1 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Binding affinity against retinoic Acid beta receptor using [3H]- -9-cis-Retinoic Acid in competitive binding assayMore data for this Ligand-Target Pair
Affinity DataEC50: >0.00100nMAssay Description:Transcriptional activation of Retinoid X receptor RXR alphaMore data for this Ligand-Target Pair
Affinity DataEC50: 21nMAssay Description:Transcriptional activation of Retinoic acid receptor RAR alphaMore data for this Ligand-Target Pair
Affinity DataEC50: 2.40nMAssay Description:Transcriptional activation of Retinoic acid receptor RAR gammaMore data for this Ligand-Target Pair
Affinity DataEC50: 4nMAssay Description:Transcriptional activation of Retinoic acid receptor RAR betaMore data for this Ligand-Target Pair
Affinity DataEC50: 24nMAssay Description:Effective concentration against RAR-gamma receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 6nMAssay Description:Effective concentration against Retinoic acid receptor betaMore data for this Ligand-Target Pair
Affinity DataEC50: 85nMAssay Description:Effective concentration against RAR-alpha receptorMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+3nMAssay Description:Binding affinity against retinoic Acid X gamma receptor using [3H]- -9-cis-Retinoic Acid in competitive binding assayMore data for this Ligand-Target Pair
Affinity DataEC50: >1.00E+4nMAssay Description:Binding affinity against retinoic Acid X alpha receptors cotransfected into CV-1 cellsMore data for this Ligand-Target Pair
Affinity DataEC50: 3nMAssay Description:Binding affinity against retinoic Acid beta receptors cotransfected into CV-1 cellsMore data for this Ligand-Target Pair
Affinity DataEC50: 30nMAssay Description:Binding affinity against retinoic Acid alpha receptors cotransfected into CV-1 cellsMore data for this Ligand-Target Pair
Affinity DataEC50: >1.00E+4nMAssay Description:Binding affinity against retinoic Acid X beta receptors cotransfected into CV-1 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 26nMAssay Description:Binding affinity against retinoic Acid gamma receptor using [3H]- -9-cis-Retinoic Acid in competitive binding assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.180nMAssay Description:Agonist activity at human RARalpha expressed in HEK293 cells by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 9.00E+3nMAssay Description:Inverse agonist activity at human RoRc-LBD fusion protein with GST expressed in BL-21 (BL3) cells assessed as SRC1 coactivator peptide recruitmentMore data for this Ligand-Target Pair
Affinity DataKd: 26nMAssay Description:Dissociation constant for Retinoic acid receptor gammaMore data for this Ligand-Target Pair
Affinity DataEC50: 2nMAssay Description:Agonist activity for retinoic acid receptor RAR gamma in transcriptional activation assayMore data for this Ligand-Target Pair
Affinity DataEC50: 30nMAssay Description:Agonist activity for retinoic acid receptor RAR alpha in transcriptional activation assayMore data for this Ligand-Target Pair
Affinity DataEC50: 3nMAssay Description:Agonist activity for retinoic acid receptor RAR beta in transcriptional activation assayMore data for this Ligand-Target Pair
Affinity DataEC50: 2.40nMAssay Description:Transcriptional activation of retinoic acid receptor RAR gammaMore data for this Ligand-Target Pair
Affinity DataEC50: 4nMAssay Description:Transcriptional activation of retinoic acid receptor RAR betaMore data for this Ligand-Target Pair
Affinity DataEC50: 21nMAssay Description:Transcriptional activation of retinoic acid receptor RAR alphaMore data for this Ligand-Target Pair
Affinity DataEC50: 1.00E+4nMAssay Description:Transcriptional activation of retinoid X receptor RXR alpha; not activeMore data for this Ligand-Target Pair
Affinity DataEC50: 2.40nMAssay Description:Transcriptional activation for RAR gamma receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 21nMAssay Description:Transcriptional activation for RAR alpha receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 4nMAssay Description:Transcriptional activation for RAR beta receptorMore data for this Ligand-Target Pair
Affinity DataKd: 26nMAssay Description:Binding affinity for Retinoic acid receptor gammaMore data for this Ligand-Target Pair
Affinity DataEC50: 3nMAssay Description:Transactivation potency of the compound was determined for Retinoic acid receptor betaMore data for this Ligand-Target Pair
TargetCytochrome P450 26A1(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 5.60E+3nMAssay Description:Eighteen compounds were tested as potential inhibitors of CYP26A1 and CYP26B1. The formation of 9-cis-4-OH-RA metabolite was monitored and the percen...More data for this Ligand-Target Pair
Affinity DataIC50: 36nMAssay Description:Binding affinity against retinoic Acid alpha receptor using [3H]- -9-cis-Retinoic Acid in competitive binding assayMore data for this Ligand-Target Pair
Affinity DataEC50: 30nMAssay Description:Transactivation potency of the compound was determined for Retinoic acid receptor alphaMore data for this Ligand-Target Pair
Affinity DataKd: 36nMAssay Description:Binding affinity for Retinoic acid receptor gammaMore data for this Ligand-Target Pair
Affinity DataEC50: 2nMAssay Description:Binding affinity of the compound was determined for Retinoic acid receptor alphaMore data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 3.70E+3nMAssay Description:Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ...More data for this Ligand-Target Pair
Affinity DataKd: 5nMAssay Description:Binding affinity of the compound was determined for Retinoic acid receptor betaMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)