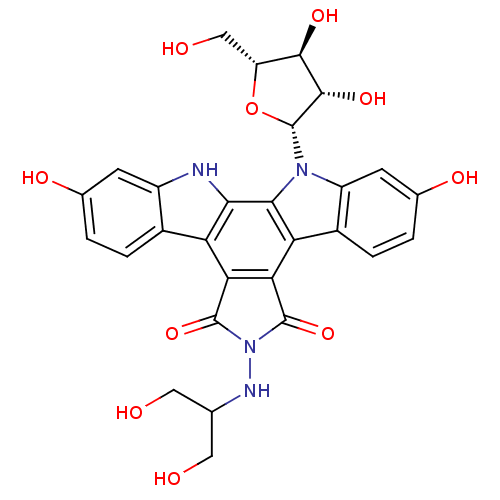
BDBM50086575 CHEMBL132533::NB-506 Analogue
SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@@H]3O[C@H](CO)[C@@H](O)[C@@H]3O)c1c1[nH]c3cc(O)ccc3c21
InChI Key InChIKey=SSNDZWKPSSKDHE-KVPHNHENSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50086575
Found 3 hits for monomerid = 50086575
Affinity DataEC50: 32nMAssay Description:Inhibitory effect on topoisomerase-I mediated DNA cleavage using supercoiled pBR322 plasmid DNAMore data for this Ligand-Target Pair
TargetO94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Curated by ChEMBL
Banyu Tsukuba Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.20E+5nMAssay Description:Inhibitory effect on protein kinase C using histone II-As as substrateMore data for this Ligand-Target Pair
TargetDNA topoisomerase 2-alpha/2-beta(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Curated by ChEMBL
Banyu Tsukuba Research Institute
Curated by ChEMBL
Affinity DataEC50: >5.00E+4nMAssay Description:Tested for inhibitory effect on topoisomerase-2 mediated DNA cleavage using super coiled pBR322 plasmid DNAMore data for this Ligand-Target Pair