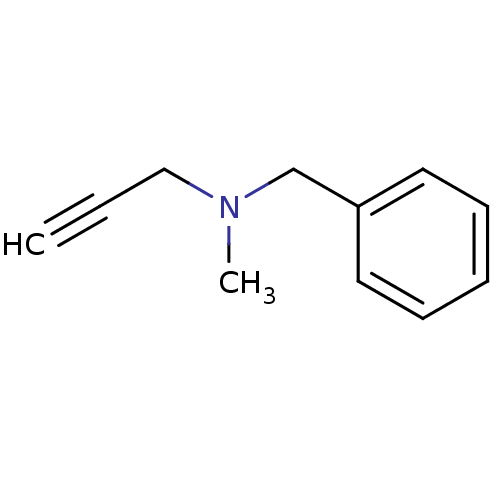
BDBM50172756 Benzyl-methyl-prop-2-ynyl-amine::CHEMBL673::Eutonyl::Eutron::N-benzyl-N-methylprop-2-yn-1-amine::PARGYLINE::US9603833, Pargyline
SMILES CN(CC#C)Cc1ccccc1
InChI Key InChIKey=DPWPWRLQFGFJFI-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 65 hits for monomerid = 50172756
Found 65 hits for monomerid = 50172756
Affinity DataKi: 20nMAssay Description:Inhibition of human recombinant microsomal MAO-B expressed in Pichia pastoris incubated for 30 mins prior to substrate addition measured after 60 min...More data for this Ligand-Target Pair
Affinity DataKi: 50nMAssay Description:Inhibition of human recombinant soluble MAO-B expressed in Pichia pastoris incubated for 30 mins prior to substrate addition measured after 60 mins b...More data for this Ligand-Target Pair
Affinity DataKi: 1.80E+3nMAssay Description:Inhibition constant against human recombinant Monoamine oxidase-B More data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] B(Rattus norvegicus (rat))
Hacettepe University
Curated by ChEMBL
Hacettepe University
Curated by ChEMBL
Affinity DataKi: 3.36E+3nMAssay Description:Inhibition of MAO-B in rat liver homogenate after 60 mins by Lineweaver-Burk plotMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Rattus norvegicus (rat))
Hacettepe University
Curated by ChEMBL
Hacettepe University
Curated by ChEMBL
Affinity DataKi: 8.50E+3nMAssay Description:Inhibition of MAO-A in rat liver homogenate after 60 mins by Lineweaver-Burk plotMore data for this Ligand-Target Pair
Affinity DataIC50: 192nMAssay Description:Inhibition of human recombinant MAO-B using p-tyramine as substrate assessed as H2O2 production after 15 mins by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataKd: 275nMAssay Description:Binding affinity to human recombinant microsomal MAO-B by ITCMore data for this Ligand-Target Pair
Affinity DataIC50: 4.10E+3nMAssay Description:Inhibition of human recombinant MAO-A using kynuramine substrate by spectrophotometric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 130nMAssay Description:Inhibition of human recombinant MAO-B using kynuramine substrate by spectrophotometric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 188nMAssay Description:Inhibition of human recombinant MAO-B using benzylamine as substrate incubated for 15 mins prior to substrate addition measured after 20 mins by fluo...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+6nMAssay Description:Inhibition of Sprague-Dawley rat Bsep expressed in plasma membrane vesicles of Sf21 cells assessed as inhibition of ATP-dependent [3H]taurocholate up...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+6nMAssay Description:Inhibition of human BSEP expressed in plasma membrane vesicles of Sf21 cells assessed as inhibition of ATP-dependent [3H]taurocholate uptakeMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] B(Rattus norvegicus (rat))
Hacettepe University
Curated by ChEMBL
Hacettepe University
Curated by ChEMBL
Affinity DataIC50: 3.85E+3nMAssay Description:Inhibition of MAOB in rat liver homogenates preincubated for 60 minsMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Rattus norvegicus (rat))
Hacettepe University
Curated by ChEMBL
Hacettepe University
Curated by ChEMBL
Affinity DataIC50: 3.90E+5nMAssay Description:Inhibition of MAOA in rat liver homogenates preincubated for 60 minsMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Rattus norvegicus (rat))
Hacettepe University
Curated by ChEMBL
Hacettepe University
Curated by ChEMBL
Affinity DataIC50: 3.90E+5nMAssay Description:Inhibition of MAO-A in rat liver homogenate after 60 mins by residual activity plotMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] B(Rattus norvegicus (rat))
Hacettepe University
Curated by ChEMBL
Hacettepe University
Curated by ChEMBL
Affinity DataIC50: 3.85E+3nMAssay Description:Inhibition of MAO-B in rat liver homogenate after 60 mins by residual activity plotMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+5nMAssay Description:Inhibition of Sprague-Dawley rat cytosolic aldehyde dehydrogenaseMore data for this Ligand-Target Pair
Affinity DataIC50: 5.60E+3nMAssay Description:Inhibition of Sprague-Dawley rat mitochondrial aldehyde dehydrogenaseMore data for this Ligand-Target Pair
Affinity DataIC50: 2.60E+3nMAssay Description:Inhibition of MAOBMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Rattus norvegicus (rat))
Hacettepe University
Curated by ChEMBL
Hacettepe University
Curated by ChEMBL
Affinity DataIC50: 3.90E+5nMAssay Description:Inhibition of rat liver MAOA after 60 mins pre-incubationMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] B(Rattus norvegicus (rat))
Hacettepe University
Curated by ChEMBL
Hacettepe University
Curated by ChEMBL
Affinity DataIC50: 2.85E+3nMAssay Description:Inhibition of rat liver MAOB after 60 mins pre-incubationMore data for this Ligand-Target Pair
Affinity DataIC50: 188nMAssay Description:Inhibition of recombinant human MAO-B using p-benzylamine substrate preincubated for 15 mins before substrate addition measured after 20 mins by fluo...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+4nMAssay Description:Inhibition of recombinant human MAO-A using kynuramine as substrate after 20 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 3.37E+3nMAssay Description:Inhibition of human recombinant microsomal MAO-A expressed in baculovirus-infected insect cells using p-tyramine as substrate preincubated for 15 min...More data for this Ligand-Target Pair
Affinity DataIC50: 195nMAssay Description:Inhibition of human recombinant microsomal MAO-B expressed in baculovirus-infected insect cells using p-tyramine as substrate preincubated for 15 min...More data for this Ligand-Target Pair
Affinity DataIC50: 95nMAssay Description:The compounds of the present invention obtained in the Production Examples were examined for the inhibitory effect on human monoamineoxydase enzymes ...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+6nMAssay Description:Inhibition of human BSEP expressed in baculovirus transfected fall armyworm Sf21 cell membranes vesicles assessed as reduction in ATP-dependent [3H]-...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+4nMAssay Description:Inhibition of recombinant human MAO-A using kynuramine as substrate by fluorescence spectroscopyMore data for this Ligand-Target Pair
Affinity DataIC50: 3.52E+3nMAssay Description:Inhibition of human MAO-A using p-tyramine as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins by fluorescenc...More data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:Inhibition of human MAO-B using p-tyramine as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins by fluorescenc...More data for this Ligand-Target Pair
Affinity DataIC50: 8.20nMAssay Description:Inhibition of MAO-B (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 11.5nMAssay Description:Inhibition of MAO-A (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.52E+3nMAssay Description:Inhibition of recombinant human MAO-A using p-tyramine as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins by...More data for this Ligand-Target Pair
Affinity DataIC50: 194nMAssay Description:Inhibition of recombinant human MAO-B using p-tyramine as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins by...More data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+3nMAssay Description:Inhibition of human recombinant MAOA using kynuramine as substrate preincubated for 30 mins followed by substrate additionMore data for this Ligand-Target Pair
Affinity DataIC50: 120nMAssay Description:Inhibition of human recombinant MAOB using benzylamine as substrate preincubated for 30 mins followed by substrate additionMore data for this Ligand-Target Pair
Affinity DataIC50: 188nMAssay Description:Inhibition of recombinant human MAOB using benzylamine as substrate preincubated for 15 mins followed by substrate addition measured after 15 mins by...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+4nMAssay Description:Inhibition of human recombinant monoamine oxidase-A assessed as kynuramine conversion to 6-hydroxyquinoline after 20 mins by fluorescence spectrophot...More data for this Ligand-Target Pair
Affinity DataIC50: 86nMAssay Description:Inhibition of recombinant human MAO-B expressed in baculovirus infected BTI insect cells incubated for 15 mins with substrate p-Tyramine by fluoresce...More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of human recombinant MAOB using kynuramine as substrate incubated for 10 mins by UPLC-ESI-MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 196nMAssay Description:Inhibition of recombinant human microsomal MAOB expressed in baculovirus infected BTI insect cells using p-tyramine as substrate preincubated for 15 ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.97E+3nMAssay Description:Inhibition of recombinant human microsomal MAOA expressed in baculovirus infected BTI insect cells using p-tyramine as substrate preincubated for 15 ...More data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] B(Rattus norvegicus (rat))
Hacettepe University
Curated by ChEMBL
Hacettepe University
Curated by ChEMBL
Affinity DataIC50: 220nMAssay Description:Inhibition of Wistar rat brain MAOB using kynuramine as substrate preincubated for 15 mins followed by substrate addition and measured after 15 mins ...More data for this Ligand-Target Pair
Affinity DataIC50: 150nMAssay Description:Inhibition of human recombinant microsomal MAO-B expressed in baculovirus infected BTI-TN-5B1-4 cells assessed as reduction in production of H202 usi...More data for this Ligand-Target Pair
TargetPyrroline-5-carboxylate reductase 1, mitochondrial(Homo sapiens)
University Of Strathclyde
Curated by ChEMBL
University Of Strathclyde
Curated by ChEMBL
Affinity DataIC50: 1.98E+5nMAssay Description:Inhibition of 6x-His-tagged and SUMO tagged human PYCR1 expressed in Escherichia coli BL21 (DE3) cells assessed as reduction in NADH oxidation incuba...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Inhibition of human recombinant MAO-B expressed in baculovirus infected BTI insect cells using benzylamine as substrate after 30 mins by spectrophoto...More data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of human recombinant MAO-A expressed in baculovirus infected BTI insect cells using kynuramine as substrate after 20 mins by spectrophotom...More data for this Ligand-Target Pair
Affinity DataIC50: 2.69E+3nMAssay Description:Inhibition of human recombinant MAOB expressed in microsomes of baculovirus-infected insect cell using kynuramine as substrate preincubated for 20 mi...More data for this Ligand-Target Pair