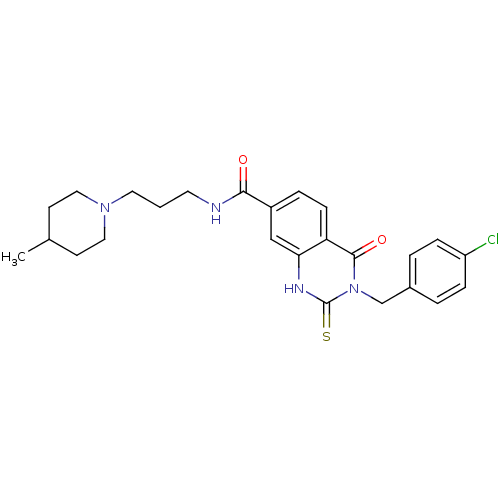
BDBM50197623 3-(4-chlorobenzyl)-N-(3-(4-methylpiperidin-1-yl)propyl)-4-oxo-2-thioxo-1,2,3,4-tetrahydroquinazoline-7-carboxamide::CHEMBL226876::cid_6616154
SMILES CC1CCN(CCCNC(=O)c2ccc3c(c2)[nH]c(=S)n(Cc2ccc(Cl)cc2)c3=O)CC1
InChI Key InChIKey=DBDVPOWGJBLQMN-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50197623
Found 3 hits for monomerid = 50197623
TargetUbiquitin-conjugating enzyme E2 N(Homo sapiens (Human))
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: >2.00E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego CA...More data for this Ligand-Target Pair
TargetVoltage-dependent T-type calcium channel subunit alpha-1G(Homo sapiens (Human))
Institute Of Science And Technology
Curated by ChEMBL
Institute Of Science And Technology
Curated by ChEMBL
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibition of alpha1G T-type calcium channel expressed in HEK293 cells by electrophysiological methodMore data for this Ligand-Target Pair
TargetNeurotensin receptor type 1(Homo sapiens (Human))
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataEC50: 1.79E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair