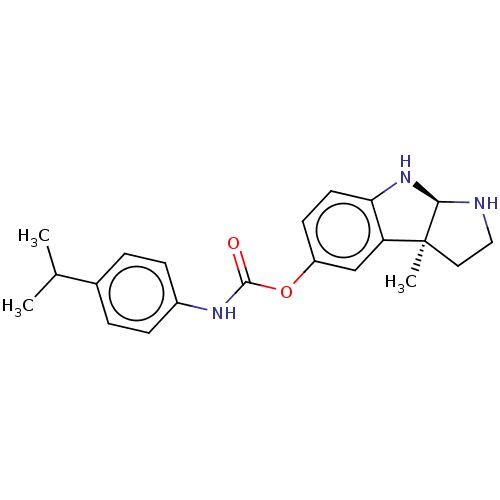
BDBM50234795 CHEMBL4089082
SMILES [H][C@]12NCC[C@@]1(C)c1cc(OC(=O)Nc3ccc(cc3)C(C)C)ccc1N2
InChI Key InChIKey=ZIGIADNCAWZUAB-CTNGQTDRSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 50234795
Found 5 hits for monomerid = 50234795
TargetCholinesterase(Homo sapiens (Human))
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Affinity DataKi: 0.131nMAssay Description:Inhibition of human serum BChE using butyrylthiocholine iodide as substrate by Ellman's methodMore data for this Ligand-Target Pair
TargetCholinesterase(Homo sapiens (Human))
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of human erythrocyte BChE using S-butyrylthiocholine as substrate preincubated for 30 mins followed by substrate addition measured after 2...More data for this Ligand-Target Pair
TargetCholinesterase(Homo sapiens (Human))
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of BChE (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 110nMAssay Description:Inhibition of AChE (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 110nMAssay Description:Inhibition of human plasma AChE using acetyl-(beta-methyl)thiocholine as substrate preincubated for 30 mins followed by substrate addition measured a...More data for this Ligand-Target Pair