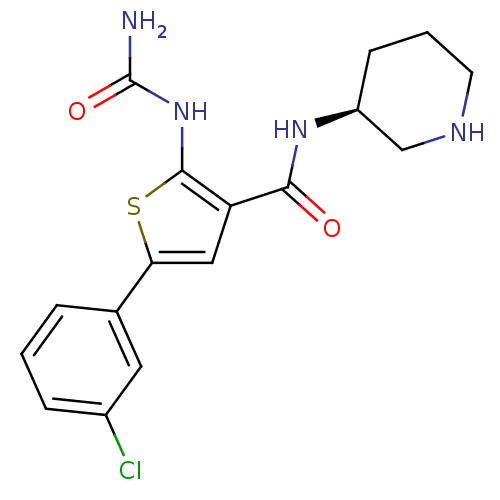
BDBM50242893 1-(3-((S)-piperidin-3-ylcarbamoyl)-5-(3-chlorophenyl)thiophen-2-yl)urea::CHEMBL2070703::CHEMBL513377
SMILES NC(=O)Nc1sc(cc1C(=O)N[C@H]1CCCNC1)-c1cccc(Cl)c1
InChI Key InChIKey=PZPQGJALDKOXRC-LBPRGKRZSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 50242893
Found 5 hits for monomerid = 50242893
Affinity DataEC50: 1.20E+3nMAssay Description:Inhibition of CHK1 in human HT29 cells assessed as check point abrogationMore data for this Ligand-Target Pair
Affinity DataEC50: 40nMAssay Description:Inhibition of CHK1 in human HT29 cells assessed as abrogation of campothecin induced check pointMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 2-associated protein 1(Homo sapiens (Human))
Astrazeneca R&D Boston
Curated by ChEMBL
Astrazeneca R&D Boston
Curated by ChEMBL
Affinity DataIC50: 8.80E+3nMAssay Description:Inhibition of Cdk1More data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:Inhibition of human recombinant CHK1 expressed in insect cells using biotinylaminohexanoyl-KKVSRSGLYRSPMPENLNRPR as substrate after 2 hrs by scintill...More data for this Ligand-Target Pair