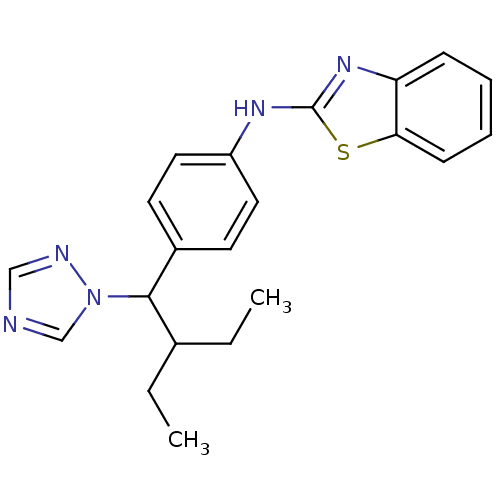
BDBM50253810 CHEMBL459505::N-(4-(2-ethyl-1-(1H-1,2,4-triazol-1-yl)butyl)phenyl)benzo[d]thiazol-2-amine::R-115866::R115866::US9963439, Talarozole
SMILES CCC(CC)C(c1ccc(Nc2nc3ccccc3s2)cc1)n1cncn1
InChI Key InChIKey=SNFYYXUGUBUECJ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50253810
Found 8 hits for monomerid = 50253810
Affinity DataEC50: 5nMAssay Description:The imidazole derivatives were evaluated for their retinoic acid metabolism inhibitory activity using a MCF-7 cell assay, using radiolabelled all-tra...More data for this Ligand-Target Pair
Affinity DataIC50: 5.10nMAssay Description:Inhibition of microsomal fraction of human CYP26A1 expressed in Sf9 cells using 9-cis-RA as substrate preincubated for 5 mins followed by NADPH addit...More data for this Ligand-Target Pair
Affinity DataIC50: 0.460nMAssay Description:Inhibition of microsomal fraction of human CYP26B1 expressed in Sf9 cells using 9-cis-RA as substrate preincubated for 5 mins followed by NADPH addit...More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 470nMAssay Description:Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.460nMAssay Description:Eighteen compounds were tested as potential inhibitors of CYP26A1 and CYP26B1. The formation of 9-cis-4-OH-RA metabolite was monitored and the percen...More data for this Ligand-Target Pair
TargetCytochrome P450 2C8(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 220nMAssay Description:Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ...More data for this Ligand-Target Pair
TargetCytochrome P450 2C9(Homo sapiens (Human))
University Of Washington Through Its Center For Commercialization
US Patent
University Of Washington Through Its Center For Commercialization
US Patent
Affinity DataIC50: 680nMAssay Description:Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.10nMAssay Description:Eighteen compounds were tested as potential inhibitors of CYP26A1 and CYP26B1. The formation of 9-cis-4-OH-RA metabolite was monitored and the percen...More data for this Ligand-Target Pair