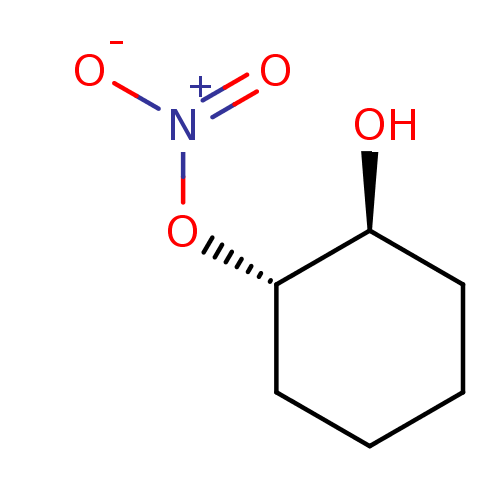
BDBM50293659 CHEMBL562358::trans-(1S(R),2S(R))-2-Hydroxycyclohexyl nitrate::trans-(1S(R),2S(R))-2-Hydroxycyclohexylnitrate
SMILES O[C@H]1CCCC[C@@H]1O[N+]([O-])=O
InChI Key InChIKey=GVSCAKVHXWAQQJ-WDSKDSINSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50293659
Found 4 hits for monomerid = 50293659
Affinity DataKi: 4.37E+4nMAssay Description:Inhibition of human erythrocyte glutathione reductaseMore data for this Ligand-Target Pair
Affinity DataKi: 9.86E+5nMAssay Description:Noncompetitive inhibition of human carbonic anhydrase 2 esterase activity by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 2.21E+8nMAssay Description:Noncompetitive inhibition of human carbonic anhydrase 1 esterase activity by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.10E+4nMAssay Description:Inhibition of human erythrocyte glutathione reductaseMore data for this Ligand-Target Pair