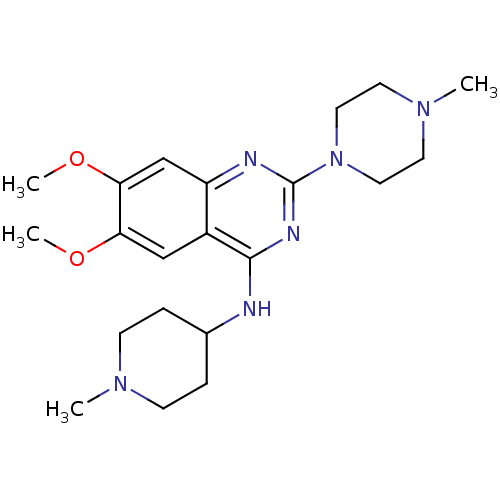
BDBM50300030 6,7-Dimaethoxy-2-(4-methylpiperazin-1-yl)-N-(1-methylpiperidin-4-yl)quinazolin-4-amine::6,7-dimethoxy-2-(4-methylpiperazin-1-yl)-N-(1-methylpiperidin-4-yl)quinazolin-4-amine::CHEMBL572373
SMILES COc1cc2nc(nc(NC3CCN(C)CC3)c2cc1OC)N1CCN(C)CC1
InChI Key InChIKey=IZHGEBMDDQJOEW-UHFFFAOYSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50300030
Found 4 hits for monomerid = 50300030
TargetHistone-lysine N-methyltransferase EHMT2(Homo sapiens (Human))
University Of North Carolina
Curated by ChEMBL
University Of North Carolina
Curated by ChEMBL
Affinity DataIC50: 200nMAssay Description:Inhibition of G9a by Alpha screen assayMore data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EHMT2(Homo sapiens (Human))
University Of North Carolina
Curated by ChEMBL
University Of North Carolina
Curated by ChEMBL
Affinity DataIC50: 1.50E+5nMAssay Description:Activity at methyl transferase activity G9a by enzyme coupled S-adenocylehomocystein detection assayMore data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EHMT2(Homo sapiens (Human))
University Of North Carolina
Curated by ChEMBL
University Of North Carolina
Curated by ChEMBL
Affinity DataIC50: 680nMAssay Description:Inhibition of G9a by Alpha screen assayMore data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EHMT2(Homo sapiens (Human))
University Of North Carolina
Curated by ChEMBL
University Of North Carolina
Curated by ChEMBL
Affinity DataIC50: 2.00E+5nMAssay Description:Inhibition of methyl transferase activity of G9a assessed as inhibition of H3K9 methylation by chemiluminescence based oxygen tunneling assayMore data for this Ligand-Target Pair