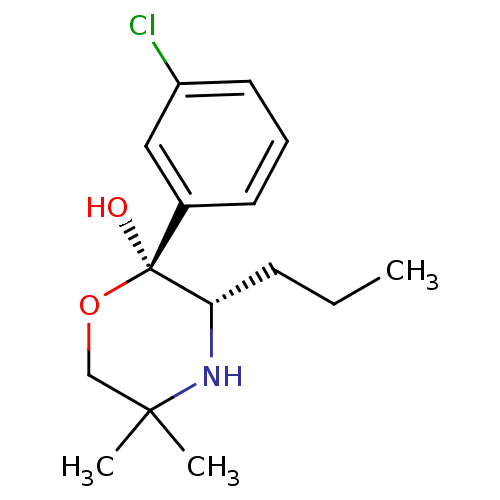
BDBM50322512 (2S,3S)-2-(3-Chlorophenyl)-5,5-dimethyl-3-propyl-morpholin-2-ol::CHEMBL1173597
SMILES CCC[C@@H]1NC(C)(C)CO[C@@]1(O)c1cccc(Cl)c1
InChI Key InChIKey=ZWMHSADWUIOURG-ZFWWWQNUSA-N
Data 13 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 13 hits for monomerid = 50322512
Found 13 hits for monomerid = 50322512
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Barrow Neurological Institute
Curated by ChEMBL
Barrow Neurological Institute
Curated by ChEMBL
Affinity DataIC50: 4.13E+3nMAssay Description:Inhibition of [3H]serotonin reuptake at human SERT expressed in HEK293 cells after 90 mins by scintillation countingMore data for this Ligand-Target Pair
TargetSodium-dependent noradrenaline transporter(Homo sapiens (Human))
Barrow Neurological Institute
Curated by ChEMBL
Barrow Neurological Institute
Curated by ChEMBL
Affinity DataIC50: 31nMAssay Description:Inhibition of [3H]norepinephrine reuptake at human NET expressed in HEK293 cells after 90 mins by scintillation countingMore data for this Ligand-Target Pair
TargetNeuronal acetylcholine receptor subunit alpha-3/beta-4(Homo sapiens (Human))
Barrow Neurological Institute
Curated by ChEMBL
Barrow Neurological Institute
Curated by ChEMBL
Affinity DataIC50: 4.80E+3nMAssay Description:Antagonist activity at alpha3beta4 nicotinic receptor in human SH-SY5Y cells assessed as inhibition varbamylcholine-induced radiolabeled Rb+ influx a...More data for this Ligand-Target Pair
TargetNeuronal acetylcholine receptor subunit alpha-4/beta-2(Homo sapiens (Human))
Barrow Neurological Institute
Curated by ChEMBL
Barrow Neurological Institute
Curated by ChEMBL
Affinity DataIC50: 7.50E+3nMAssay Description:Antagonist activity at alpha4beta2 nicotinic receptor in human SH-EP1 cells assessed as inhibition varbamylcholine-induced radiolabeled Rb+ influx at...More data for this Ligand-Target Pair
TargetNeuronal acetylcholine receptor subunit alpha-4/beta-4(Homo sapiens (Human))
Barrow Neurological Institute
Curated by ChEMBL
Barrow Neurological Institute
Curated by ChEMBL
Affinity DataIC50: 1.80E+4nMAssay Description:Antagonist activity at alpha4beta4 nicotinic receptor in human SH-EP1 cells assessed as inhibition varbamylcholine-induced radiolabeled Rb+ influx at...More data for this Ligand-Target Pair
TargetAcetylcholine receptor subunit alpha/beta/delta/gamma(Homo sapiens (Human))
Barrow Neurological Institute
Curated by ChEMBL
Barrow Neurological Institute
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Antagonist activity at alpha-1-beta-1-gamma-delta nicotinic receptor in human TE671 cells assessed as inhibition varbamylcholine-induced radiolabeled...More data for this Ligand-Target Pair
TargetNeuronal acetylcholine receptor subunit alpha-4/beta-4(Homo sapiens (Human))
Barrow Neurological Institute
Curated by ChEMBL
Barrow Neurological Institute
Curated by ChEMBL
Affinity DataIC50: 8.00E+3nMAssay Description:Antagonist activity at human alpha4beta4 nAChR in HEK293 cells assessed as inhibition of carbamylcholine-induced radiolabeled Rb ion effluxMore data for this Ligand-Target Pair
TargetSodium-dependent dopamine transporter(Homo sapiens (Human))
Research Triangle Institute
Curated by ChEMBL
Research Triangle Institute
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibition of human DAT in HEK293 cells assessed as inhibition of [3H]dopamine uptakeMore data for this Ligand-Target Pair
TargetSodium-dependent noradrenaline transporter(Homo sapiens (Human))
Barrow Neurological Institute
Curated by ChEMBL
Barrow Neurological Institute
Curated by ChEMBL
Affinity DataIC50: 9nMAssay Description:Inhibition of human NET in HEK293 cells assessed as inhibition of [3H]norepinephrine uptakeMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Barrow Neurological Institute
Curated by ChEMBL
Barrow Neurological Institute
Curated by ChEMBL
Affinity DataIC50: 300nMAssay Description:Inhibition of human SERT in HEK293 cells assessed as inhibition of [3H]serotonin uptakeMore data for this Ligand-Target Pair
TargetNeuronal acetylcholine receptor subunit alpha-3/beta-4(Homo sapiens (Human))
Barrow Neurological Institute
Curated by ChEMBL
Barrow Neurological Institute
Curated by ChEMBL
Affinity DataIC50: 3.10E+3nMAssay Description:Antagonist activity at human alpha3beta4 nAChR in HEK293 cells assessed as inhibition of carbamylcholine-induced radiolabeled Rb ion effluxMore data for this Ligand-Target Pair
TargetNeuronal acetylcholine receptor subunit alpha-4/beta-2(Homo sapiens (Human))
Barrow Neurological Institute
Curated by ChEMBL
Barrow Neurological Institute
Curated by ChEMBL
Affinity DataIC50: 9.50E+3nMAssay Description:Antagonist activity at human alpha4beta2 nAChR in HEK293 cells assessed as inhibition of carbamylcholine-induced radiolabeled Rb ion effluxMore data for this Ligand-Target Pair
TargetSodium-dependent dopamine transporter(Homo sapiens (Human))
Research Triangle Institute
Curated by ChEMBL
Research Triangle Institute
Curated by ChEMBL
Affinity DataIC50: 30nMAssay Description:Inhibition of [3H]dopamine reuptake at human DAT expressed in HEK293 cells after 90 mins by scintillation countingMore data for this Ligand-Target Pair