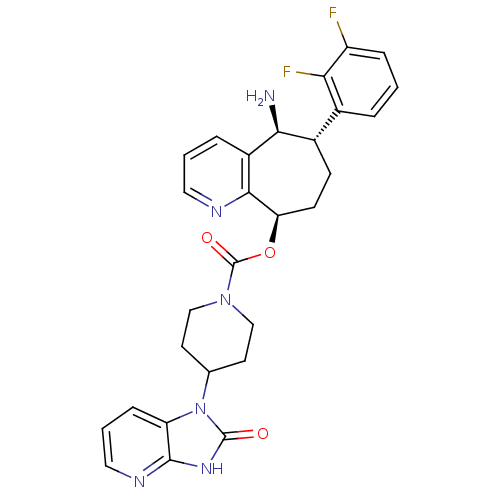
BDBM50400098 CHEMBL2178422
SMILES N[C@H]1[C@@H](CC[C@@H](OC(=O)N2CCC(CC2)n2c3cccnc3[nH]c2=O)c2ncccc12)c1cccc(F)c1F
InChI Key InChIKey=KRNAOFGYEFKHPB-ANJVHQHFSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50400098
Found 4 hits for monomerid = 50400098
TargetCalcitonin gene-related peptide type 1 receptor(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataKi: 0.0270nMAssay Description:Displacement of [125I]-CGRP from CGRP receptor in human SK-N-MC cells after 2 hrs by gamma scintillation counter analysisMore data for this Ligand-Target Pair
TargetCalcitonin gene-related peptide type 1 receptor(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataEC50: 0.140nMAssay Description:Antagonist activity at CGRP receptor in human SK-N-MC cells assessed as inhibition of CGRP-stimulated cAMP production preincubated for 15 mins prior ...More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataIC50: 1.70E+4nMAssay Description:Inhibition of human recombinant CYP3A4 using benzoylresorufin as substrate after 45 mins by fluorescence assayMore data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataIC50: 1.70E+4nMAssay Description:Inhibition of human recombinant CYP3A4 using 7-benzyloxy-4-trifluoromethylcoumarin as substrate after 20 mins by fluorescence assayMore data for this Ligand-Target Pair