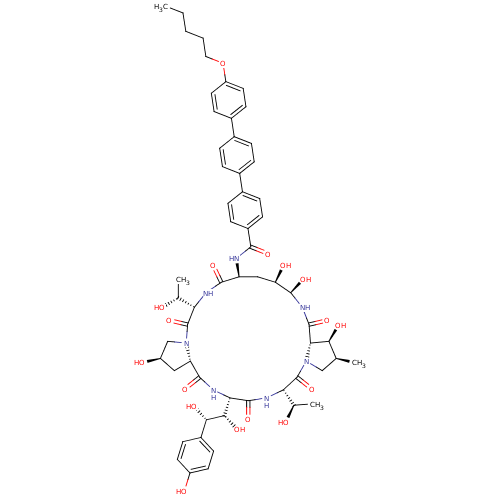
BDBM50417554 ANIDULAFUNGIN
SMILES CCCCCOc1ccc(cc1)-c1ccc(cc1)-c1ccc(cc1)C(=O)N[C@H]1C[C@@H](O)[C@@H](O)NC(=O)[C@@H]2[C@@H](O)[C@@H](C)CN2C(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@@H]2C[C@@H](O)CN2C(=O)[C@@H](NC1=O)[C@@H](C)O)[C@H](O)[C@@H](O)c1ccc(O)cc1)[C@@H](C)O
InChI Key InChIKey=JHVAMHSQVVQIOT-MFAJLEFUSA-N
Data 41 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 41 hits for monomerid = 50417554
Found 41 hits for monomerid = 50417554
Affinity DataIC50: >1.75E+4nMAssay Description:Inhibition of human CYP1A2More data for this Ligand-Target Pair
Target1,3-beta-glucan synthase(Candida tropicalis)
University Of Medicine And Dentistry Of New Jersey
Curated by ChEMBL
University Of Medicine And Dentistry Of New Jersey
Curated by ChEMBL
Affinity DataIC50: 357nMAssay Description:Inhibition of Candida tropicalis T19 blood stream isolate glucan synthase subunit FKS1p with FLTLS/PLRDP mutant at biphasic kineticsMore data for this Ligand-Target Pair
Affinity DataIC50: >1.75E+4nMAssay Description:Inhibition of human CYP2C8 using rosiglitazone as a substrateMore data for this Ligand-Target Pair
Affinity DataIC50: >1.75E+4nMAssay Description:Inhibition of human CYP2C9More data for this Ligand-Target Pair
Affinity DataIC50: >1.75E+4nMAssay Description:Inhibition of human CYP2C19More data for this Ligand-Target Pair
Affinity DataIC50: >1.75E+4nMAssay Description:Inhibition of human CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: >1.75E+4nMAssay Description:Inhibition of human CYP2B6More data for this Ligand-Target Pair
Affinity DataIC50: 4.64E+3nMAssay Description:Ten-point DRCs were generated for each drug. Vero cells were seeded at 1.2 × 104 cells per well in DMEM, supplemented with 2% FBS and 1× ...More data for this Ligand-Target Pair
Target1,3-beta-glucan synthase(Neosartorya fumigata)
Umdnj-New Jersey Medical School
Curated by ChEMBL
Umdnj-New Jersey Medical School
Curated by ChEMBL
Affinity DataIC50: 29nMAssay Description:Inhibition of 1,3-beta-D-glucan synthase EMFR-S678P mutant from Aspergillus fumigatus assessed as incorporation of [3H]glucoseMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase(Candida glabrata)
University Of Medicine And Dentistry Of New Jersey
Curated by ChEMBL
University Of Medicine And Dentistry Of New Jersey
Curated by ChEMBL
Affinity DataIC50: 3.38E+3nMAssay Description:Inhibition of Candida glabrata isolate 5847 1,3-beta-D-glucan synthase Fks1p S629P mutant/wild type Fks2pMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase(Candida glabrata)
University Of Medicine And Dentistry Of New Jersey
Curated by ChEMBL
University Of Medicine And Dentistry Of New Jersey
Curated by ChEMBL
Affinity DataIC50: 89.3nMAssay Description:Inhibition of Candida glabrata isolate 21900 1,3-beta-D-glucan synthase Fks1p D632G mutant/wild type Fks2pMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase(Candida glabrata)
University Of Medicine And Dentistry Of New Jersey
Curated by ChEMBL
University Of Medicine And Dentistry Of New Jersey
Curated by ChEMBL
Affinity DataIC50: 111nMAssay Description:Inhibition of Candida glabrata isolate 42031 1,3-beta-D-glucan synthase wild type Fks1p/Fks2p D666E mutantMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase(Candida glabrata)
University Of Medicine And Dentistry Of New Jersey
Curated by ChEMBL
University Of Medicine And Dentistry Of New Jersey
Curated by ChEMBL
Affinity DataIC50: 97nMAssay Description:Inhibition of Candida glabrata isolate 42997 1,3-beta-D-glucan synthase Fks1p F625S mutant/wild type Fks2pMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase(Candida glabrata)
University Of Medicine And Dentistry Of New Jersey
Curated by ChEMBL
University Of Medicine And Dentistry Of New Jersey
Curated by ChEMBL
Affinity DataIC50: 154nMAssay Description:Inhibition of Candida glabrata isolate 41400 1,3-beta-D-glucan synthase wild type Fks1p/Fks2p D666G mutantMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase(Candida glabrata)
University Of Medicine And Dentistry Of New Jersey
Curated by ChEMBL
University Of Medicine And Dentistry Of New Jersey
Curated by ChEMBL
Affinity DataIC50: 74.7nMAssay Description:Inhibition of Candida glabrata isolate 5416 1,3-beta-D-glucan synthase wild type Fks1p/Fks2p W1375L mutantMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase(Candida glabrata)
University Of Medicine And Dentistry Of New Jersey
Curated by ChEMBL
University Of Medicine And Dentistry Of New Jersey
Curated by ChEMBL
Affinity DataIC50: 87.3nMAssay Description:Inhibition of Candida glabrata isolate 51916 1,3-beta-D-glucan synthase wild type Fks1p/Fks2p P667T mutantMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase(Candida glabrata)
University Of Medicine And Dentistry Of New Jersey
Curated by ChEMBL
University Of Medicine And Dentistry Of New Jersey
Curated by ChEMBL
Affinity DataIC50: 132nMAssay Description:Inhibition of Candida glabrata isolate 3169 1,3-beta-D-glucan synthase Fks1p D632E mutant/wild type Fks2pMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase(Candida glabrata)
University Of Medicine And Dentistry Of New Jersey
Curated by ChEMBL
University Of Medicine And Dentistry Of New Jersey
Curated by ChEMBL
Affinity DataIC50: 752nMAssay Description:Inhibition of Candida glabrata isolate 41026 1,3-beta-D-glucan synthase wild type Fks1p/Fks2p F659S mutantMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase(Candida glabrata)
University Of Medicine And Dentistry Of New Jersey
Curated by ChEMBL
University Of Medicine And Dentistry Of New Jersey
Curated by ChEMBL
Affinity DataIC50: 75.9nMAssay Description:Inhibition of Candida glabrata isolate 234 1,3-beta-D-glucan synthase wild type Fks1p/Fks2p F659V mutantMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase(Candida glabrata)
University Of Medicine And Dentistry Of New Jersey
Curated by ChEMBL
University Of Medicine And Dentistry Of New Jersey
Curated by ChEMBL
Affinity DataIC50: 3.51E+3nMAssay Description:Inhibition of Candida glabrata isolate 3830 1,3-beta-D-glucan synthase wild type Fks1p/Fks2p S663P mutantMore data for this Ligand-Target Pair
Affinity DataIC50: 16.4nMAssay Description:Inhibition of glucan synthase in Candida albicans 36082 assessed as incorporation of [3H]uridine diphosphoglucose in presence of 20% serumMore data for this Ligand-Target Pair
Affinity DataIC50: 16.2nMAssay Description:Inhibition of glucan synthase in Candida albicans ATCC 36082More data for this Ligand-Target Pair
Affinity DataIC50: 62.7nMAssay Description:Inhibition of glucan synthase in Candida albicans ATCC 36082 in presence of 20% human serumMore data for this Ligand-Target Pair
Affinity DataIC50: 13.4nMAssay Description:Inhibition of glucan synthase in Candida albicans SC5314 in presence of 10% human serumMore data for this Ligand-Target Pair
Affinity DataIC50: 21.7nMAssay Description:Inhibition of glucan synthase in Candida albicans ATCC 90028 in presence of 50% human serumMore data for this Ligand-Target Pair
Affinity DataIC50: 25.8nMAssay Description:Inhibition of glucan synthase in Candida albicans 36082 assessed as incorporation of [3H]uridine diphosphoglucose in presence of 50% serumMore data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:Inhibition of glucan synthase in Candida albicans SC5314More data for this Ligand-Target Pair
Affinity DataIC50: 21nMAssay Description:Inhibition of glucan synthase in Candida albicans ATCC 36082 in presence of 10% human serumMore data for this Ligand-Target Pair
Affinity DataIC50: 16.3nMAssay Description:Inhibition of glucan synthase in Candida albicans ATCC 90028 in presence of 20% human serumMore data for this Ligand-Target Pair
Affinity DataIC50: 78.1nMAssay Description:Inhibition of glucan synthase in Candida albicans SC5314 in presence of 50% human serumMore data for this Ligand-Target Pair
Affinity DataIC50: 0.877nMAssay Description:Inhibition of glucan synthase in Candida albicans ATCC 90028More data for this Ligand-Target Pair
Affinity DataIC50: 4.14nMAssay Description:Inhibition of glucan synthase in Candida albicans 36082 assessed as incorporation of [3H]uridine diphosphoglucose in presence of 10% serumMore data for this Ligand-Target Pair
Affinity DataIC50: 0.880nMAssay Description:Inhibition of glucan synthase in Candida albicans 36082 assessed as incorporation of [3H]uridine diphosphoglucoseMore data for this Ligand-Target Pair
Affinity DataIC50: 65.5nMAssay Description:Inhibition of glucan synthase in Candida albicans SC5314 in presence of 20% human serumMore data for this Ligand-Target Pair
Affinity DataIC50: 4.14nMAssay Description:Inhibition of glucan synthase in Candida albicans ATCC 90028 in presence of 10% human serumMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase(Candida tropicalis)
University Of Medicine And Dentistry Of New Jersey
Curated by ChEMBL
University Of Medicine And Dentistry Of New Jersey
Curated by ChEMBL
Affinity DataIC50: 0.503nMAssay Description:Inhibition of Candida tropicalis T19 blood stream isolate glucan synthase subunit FKS1p with FLTLS/PLRDP mutant at monophasic kineticsMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase(Candida tropicalis)
University Of Medicine And Dentistry Of New Jersey
Curated by ChEMBL
University Of Medicine And Dentistry Of New Jersey
Curated by ChEMBL
Affinity DataIC50: 27.7nMAssay Description:Inhibition of Candida tropicalis T26 blood stream isolate glucan synthase subunit FKS1p with LLTLSLRDP mutant at monophasic kineticsMore data for this Ligand-Target Pair
Target1,3-beta-D-glucan synthase catalytic subunit(Candida albicans)
University Of Medicine And Dentistry Of New Jersey
Curated by ChEMBL
University Of Medicine And Dentistry Of New Jersey
Curated by ChEMBL
Affinity DataIC50: 1.60nMAssay Description:Inhibition of Candida albicans ATCC 90028 glucan synthase at monophasic kineticsMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase(Candida tropicalis)
University Of Medicine And Dentistry Of New Jersey
Curated by ChEMBL
University Of Medicine And Dentistry Of New Jersey
Curated by ChEMBL
Affinity DataIC50: 0.281nMAssay Description:Inhibition of Candida tropicalis T3 blood stream isolate glucan synthase subunit FKS1p with FLTLS/PLRDP mutant at monophasic kineticsMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase(Candida tropicalis)
University Of Medicine And Dentistry Of New Jersey
Curated by ChEMBL
University Of Medicine And Dentistry Of New Jersey
Curated by ChEMBL
Affinity DataIC50: 204nMAssay Description:Inhibition of Candida tropicalis T3 blood stream isolate glucan synthase subunit FKS1p with FLTLS/PLRDP mutant at biphasic kineticsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+4nMAssay Description:Inhibition of human CYP2C8 using amodiaquine as a substrateMore data for this Ligand-Target Pair