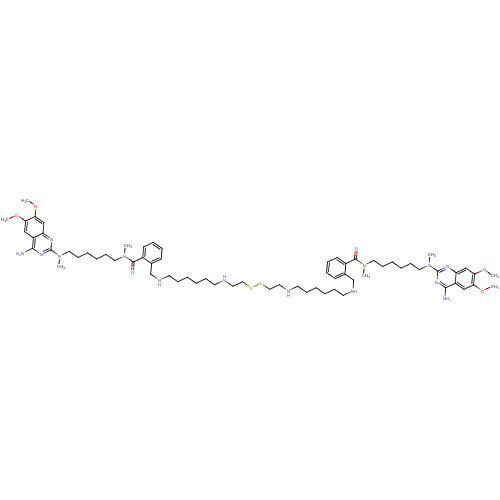
BDBM50422382 CHEMBL1203233
SMILES COc1cc2nc(nc(N)c2cc1OC)N(C)CCCCCCN(C)C(=O)c1ccccc1CNCCCCCCNCCSSCCNCCCCCCNCc1ccccc1C(=O)N(C)CCCCCCN(C)c1nc(N)c2cc(OC)c(OC)cc2n1
InChI Key InChIKey=VZDUXQGYCAXMBA-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50422382
Found 3 hits for monomerid = 50422382
Affinity DataIC50: 8.90nMAssay Description:Antagonist affinity [non competitive (irreversible)] at alpha-1D-adrenoreceptors on isolated thoracic aorta from ratMore data for this Ligand-Target Pair
Affinity DataIC50: 1.86E+3nMAssay Description:Antagonist affinity [non competitive (irreversible)] at alpha-1B-adrenoreceptors on isolated spleen from ratMore data for this Ligand-Target Pair
Affinity DataIC50: 1.86E+3nMAssay Description:Antagonist affinity [non competitive (irreversible)] at alpha-1A-adrenoreceptors on isolated prostatic Vas deferens from ratMore data for this Ligand-Target Pair