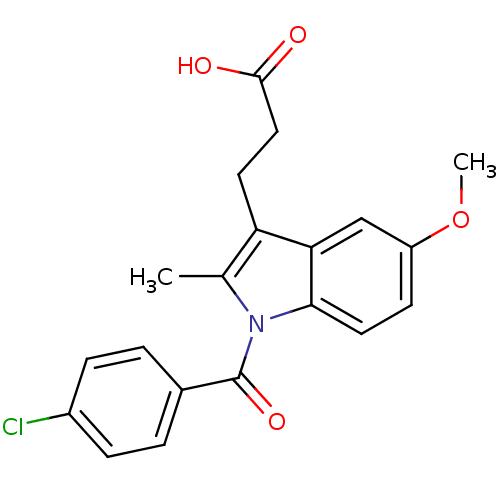
BDBM50427625 CHEMBL178687::US9346803, Table 2, Compound 6: 3-[1-(4-chlorobenzoyl)-5-methoxy-2-methyl-1H-indol-3-yl]propanoic acid
SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CCC(O)=O)c2c1
InChI Key InChIKey=ZXFPVIQVVULPBC-UHFFFAOYSA-N
Data 9 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 50427625
Found 9 hits for monomerid = 50427625
Affinity DataIC50: 0.220nMAssay Description:Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ...More data for this Ligand-Target Pair
Affinity DataIC50: >100nMAssay Description:Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ...More data for this Ligand-Target Pair
Affinity DataIC50: 57nMAssay Description:Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ...More data for this Ligand-Target Pair
Affinity DataIC50: >100nMAssay Description:Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ...More data for this Ligand-Target Pair
Affinity DataIC50: 220nMAssay Description:Inhibition of human recombinant AKR1C3-mediated NADP+-dependent oxidation of S-(+)-1,2,3,4-tetrahydro-1-naphtholMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of human recombinant AKR1C4-mediated NADP+-dependent oxidation of S-(+)-1,2,3,4-tetrahydro-1-naphtholMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of human recombinant AKR1C1-mediated NADP+-dependent oxidation of S-(+)-1,2,3,4-tetrahydro-1-naphtholMore data for this Ligand-Target Pair
Affinity DataIC50: 5.65E+4nMAssay Description:Inhibition of human recombinant AKR1C2-mediated NADP+-dependent oxidation of S-(+)-1,2,3,4-tetrahydro-1-naphtholMore data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 1(Homo sapiens (Human))
Vanderbilt University School Of Medicine
Curated by ChEMBL
Vanderbilt University School Of Medicine
Curated by ChEMBL
Affinity DataIC50: 4.88E+4nMAssay Description:Inhibition of COX1 (unknown origin)-mediated oxidation of N,N,N,Ntetramethyl-1,4-phenylenediamine using arachidonic acid as substrate by colorimetric...More data for this Ligand-Target Pair