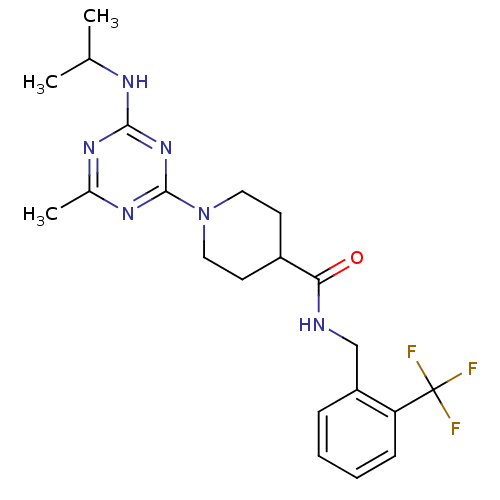
BDBM50435755 CHEMBL2392696
SMILES CC(C)Nc1nc(C)nc(n1)N1CCC(CC1)C(=O)NCc1ccccc1C(F)(F)F
InChI Key InChIKey=JQHCYQUHJQOVNX-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50435755
Found 3 hits for monomerid = 50435755
Affinity DataIC50: 1nMAssay Description:Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of human soluble epoxide hydrolase overexpressed in HEK293F cells using EET as substrate assessed as formation of DHET incubated for 30 mi...More data for this Ligand-Target Pair
Affinity DataIC50: 400nMAssay Description:Inhibition of CYP2C9 (unknown origin)More data for this Ligand-Target Pair