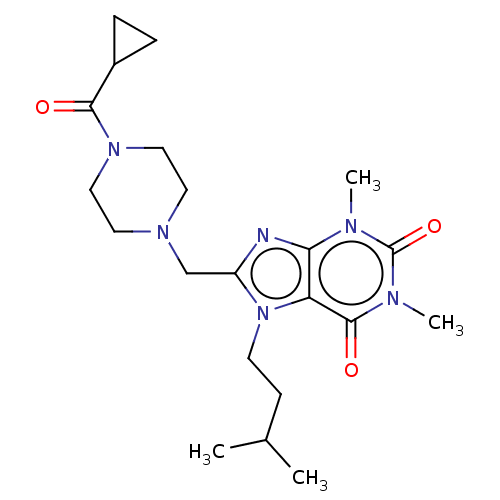
BDBM50456226 CHEMBL4215649
SMILES CC(C)CCn1c(CN2CCN(CC2)C(=O)C2CC2)nc2n(C)c(=O)n(C)c(=O)c12
InChI Key InChIKey=FSXIBBYWVGWQJL-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50456226
Found 3 hits for monomerid = 50456226
TargetAldehyde dehydrogenase 1A1(Homo sapiens (Human))
National Center For Advancing Translational Sciences
Curated by ChEMBL
National Center For Advancing Translational Sciences
Curated by ChEMBL
Affinity DataEC50: 7.79E+3nMAssay Description:Inhibition of ALDH1A1 in human SKOV3TR cells assessed as potentiation of 100 nM paclitaxel-mediated cytotoxicity after 4 days by CellTiter-Glo assayMore data for this Ligand-Target Pair
TargetAldehyde dehydrogenase 1A1(Homo sapiens (Human))
National Center For Advancing Translational Sciences
Curated by ChEMBL
National Center For Advancing Translational Sciences
Curated by ChEMBL
Affinity DataIC50: 41nMAssay Description:Inhibition of human ALDH1A1 using NAD+/propionaldehyde as substrate/cofactor preincubated for 15 mins followed by substrate/cofactor addition measure...More data for this Ligand-Target Pair
TargetAldehyde dehydrogenase 1A1(Homo sapiens (Human))
National Center For Advancing Translational Sciences
Curated by ChEMBL
National Center For Advancing Translational Sciences
Curated by ChEMBL
Affinity DataIC50: 6.79E+3nMAssay Description:Inhibition of ALDH1A1 in human MIAPaCa2 cells after 30 mins by aldefluor assayMore data for this Ligand-Target Pair