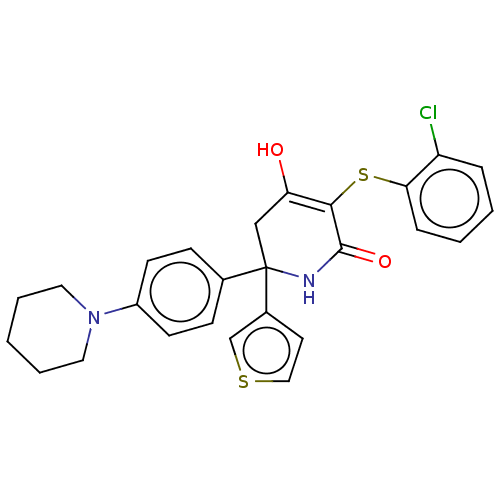
BDBM50534330 CHEMBL4520899
SMILES OC1=C(Sc2ccccc2Cl)C(=O)NC(C1)(c1ccsc1)c1ccc(cc1)N1CCCCC1
InChI Key InChIKey=OSCRHRYASAHIKU-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50534330
Found 3 hits for monomerid = 50534330
Affinity DataIC50: 49nMAssay Description:Inhibition of human recombinant carboxy-terminal His-tagged LDHB by UV endpoint assayMore data for this Ligand-Target Pair
Affinity DataIC50: 670nMAssay Description:Inhibition of LDHA in human MiPaca2 cells assessed as inhibition of lactate productionMore data for this Ligand-Target Pair
Affinity DataIC50: 9nMAssay Description:Inhibition of human recombinant carboxy-terminal His-tagged LDHA by UV endpoint assayMore data for this Ligand-Target Pair