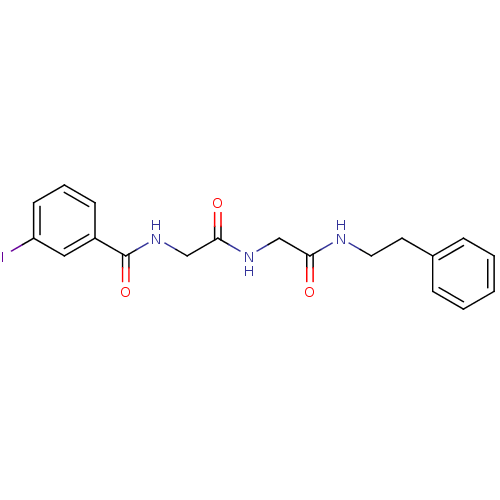
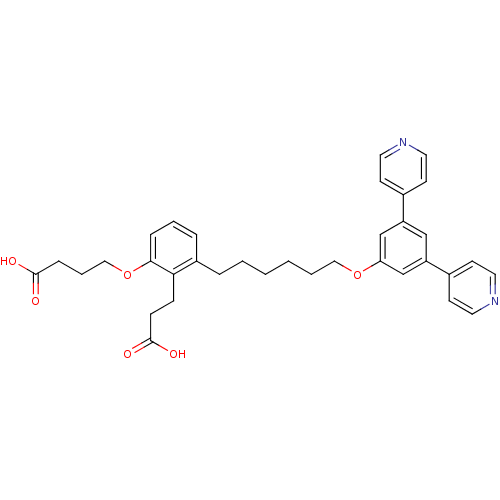
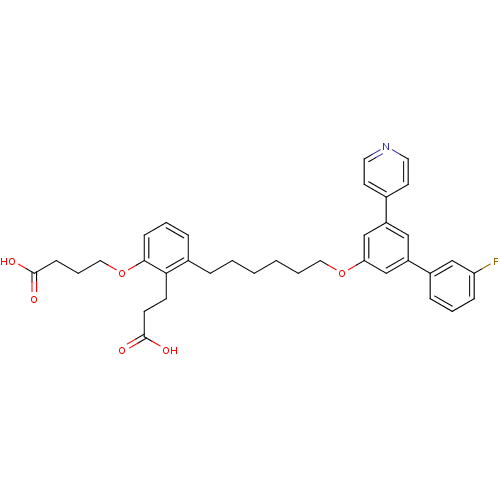
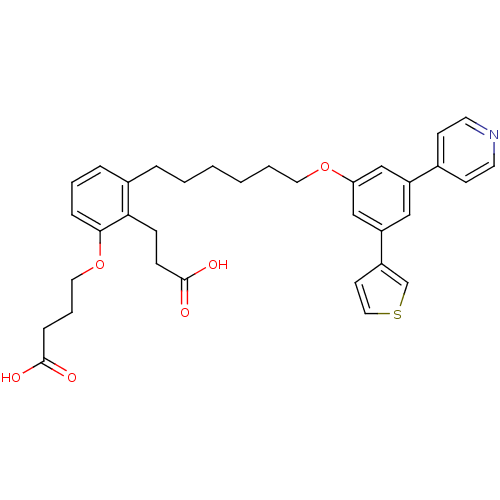
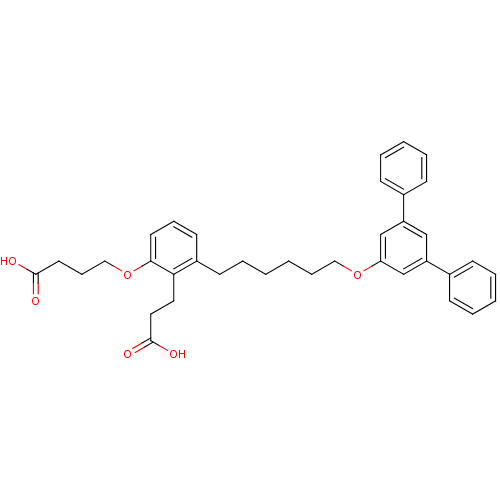
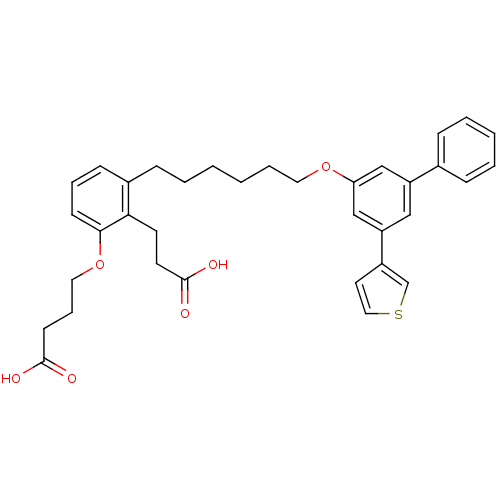
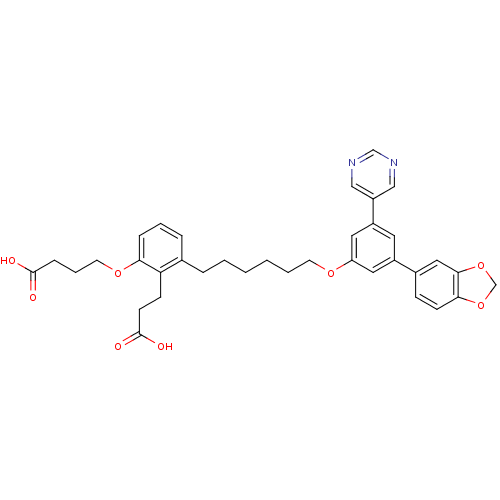
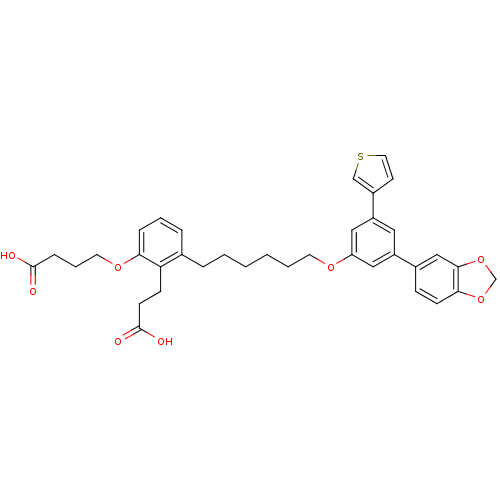
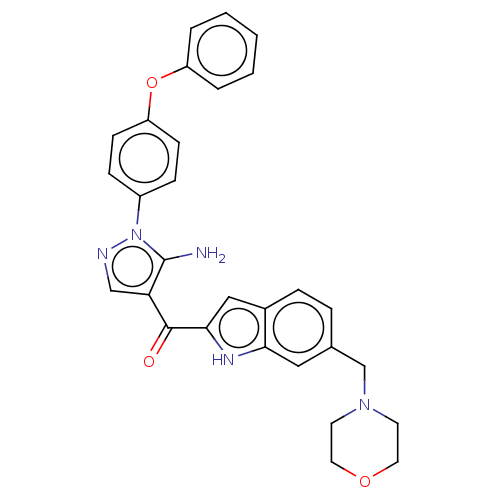
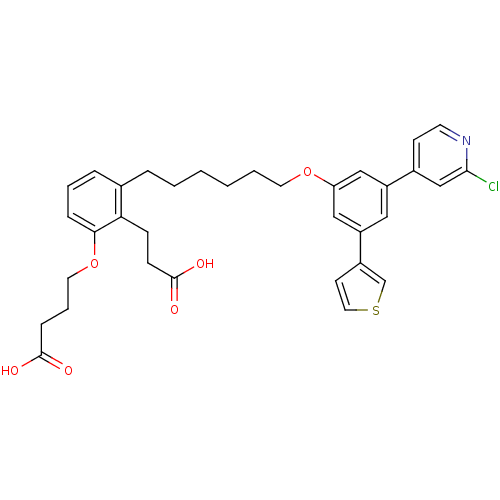
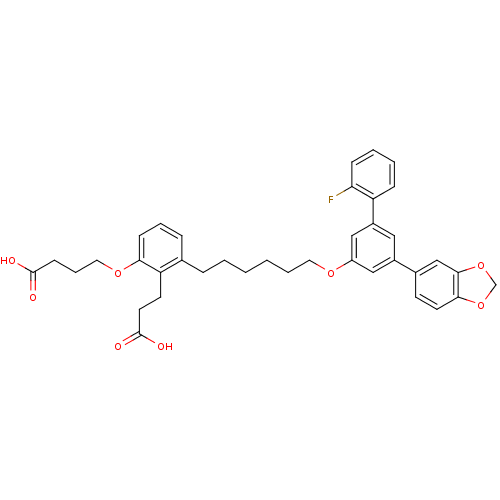
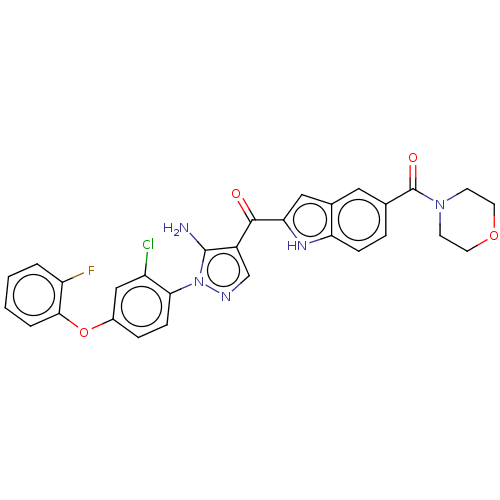
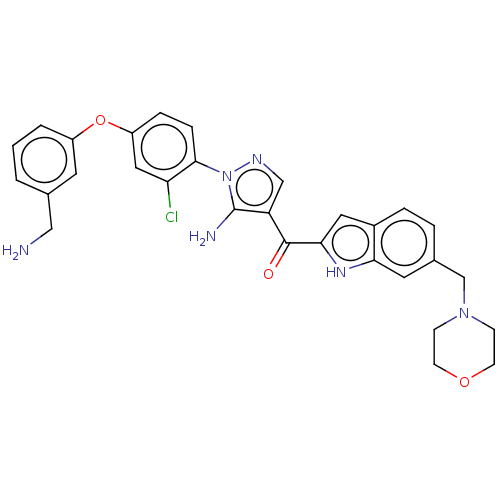
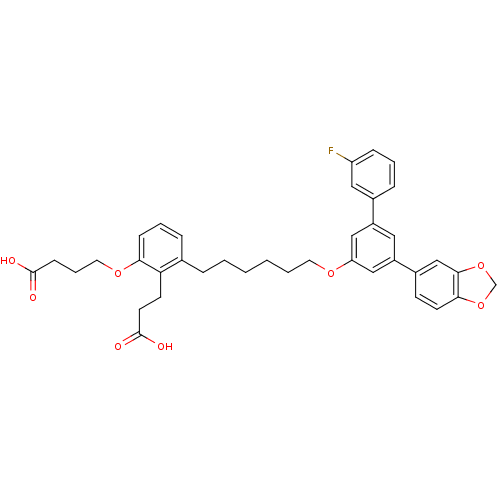
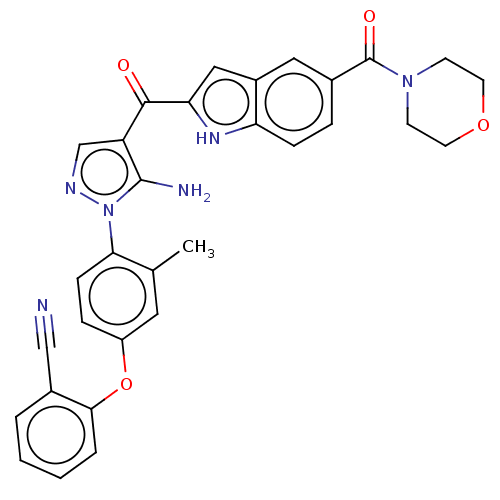
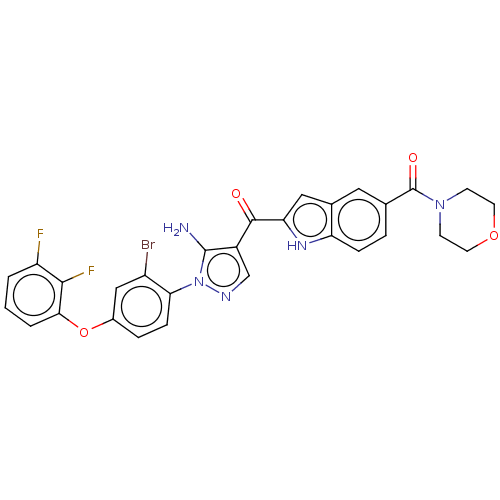
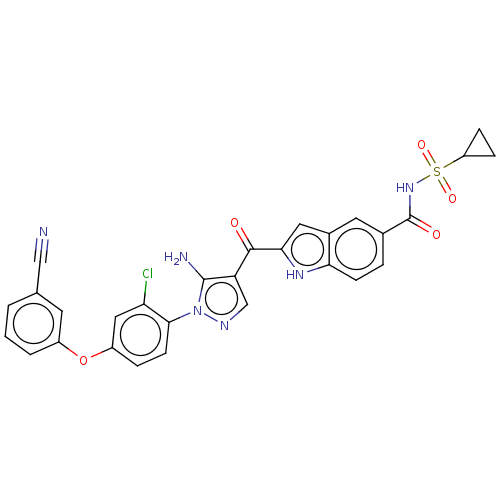
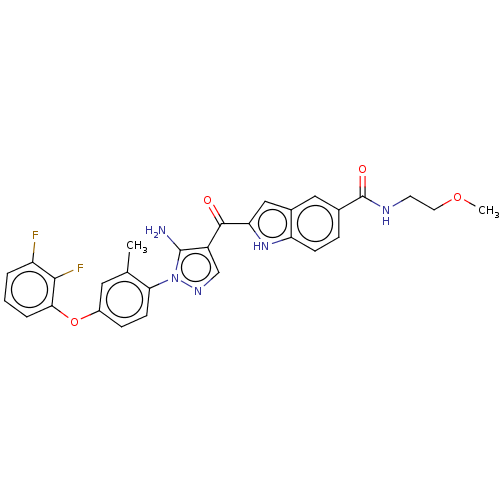
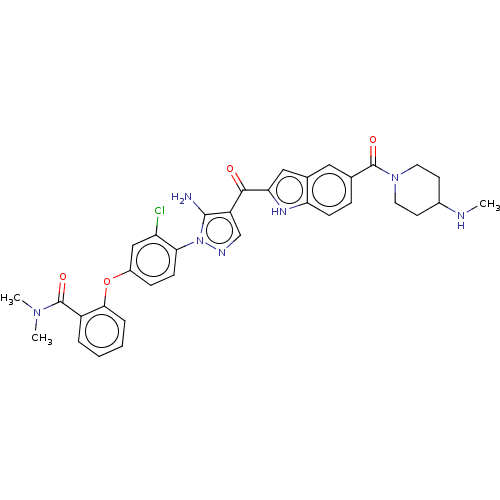
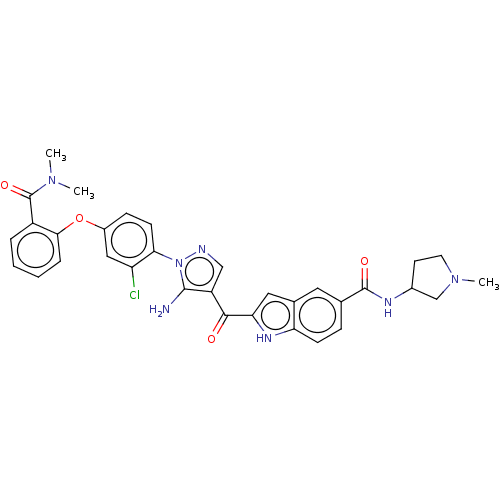
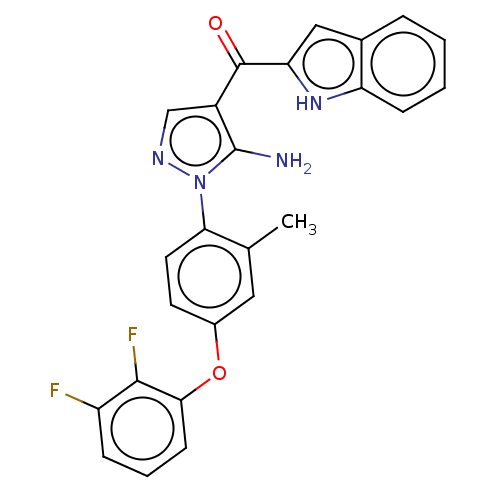
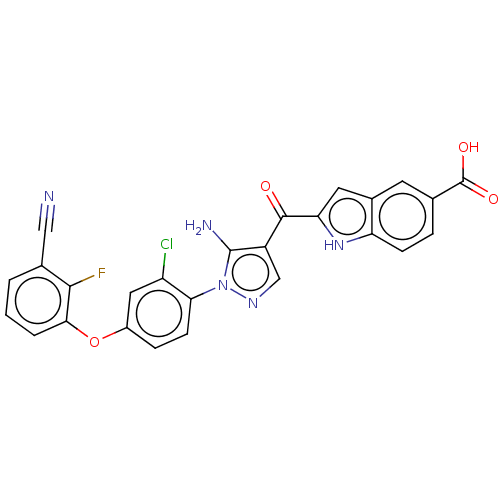
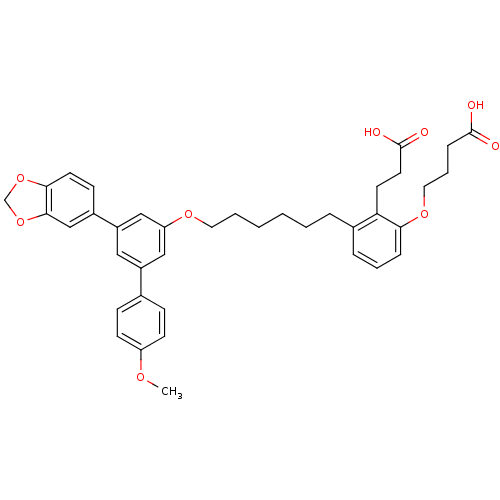
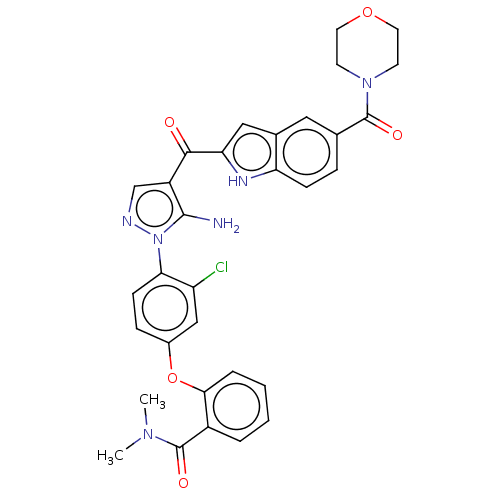
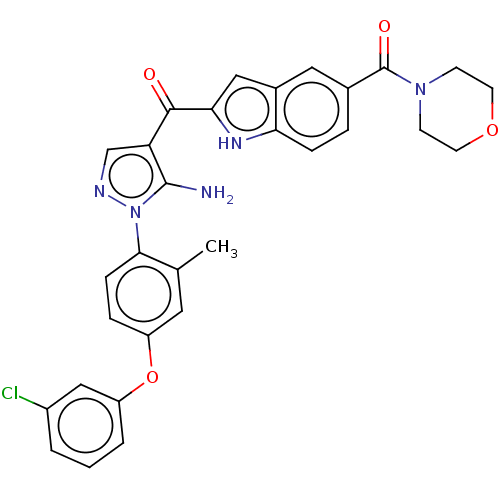
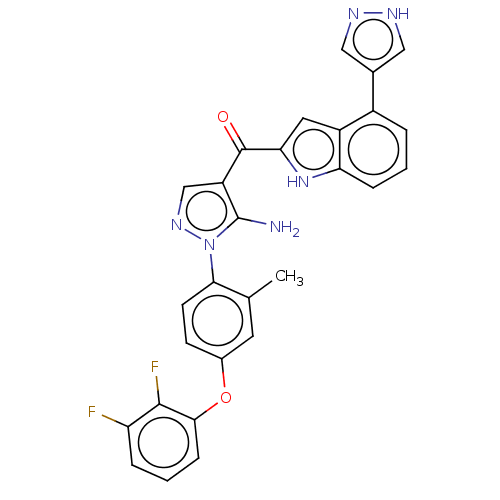
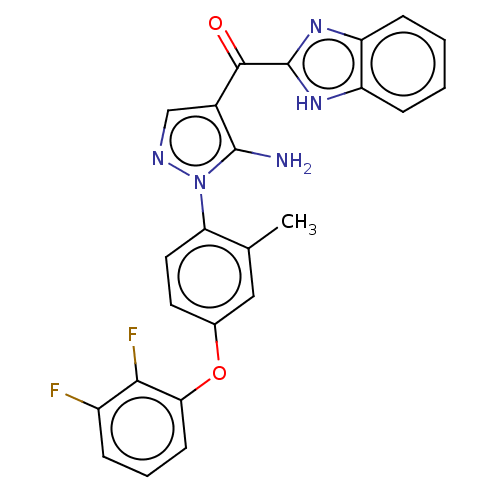
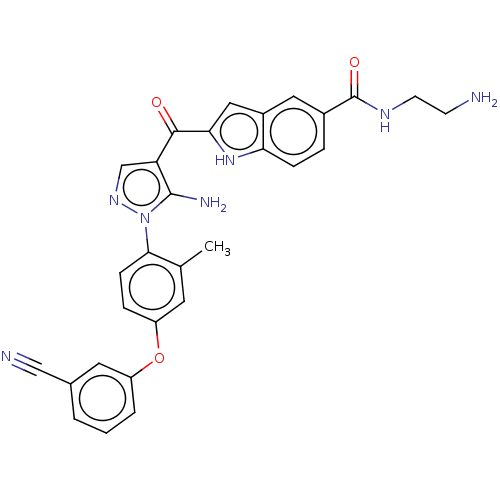
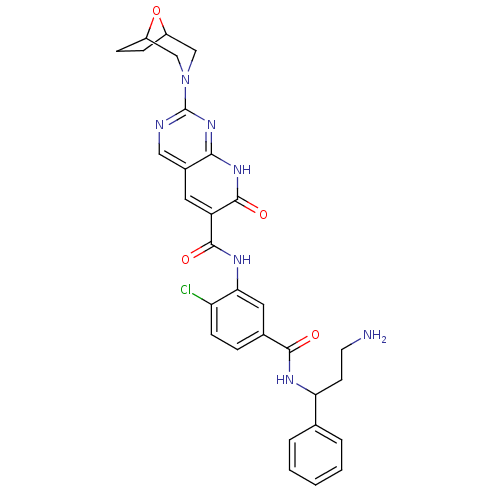
TargetBifunctional purine biosynthesis protein ATIC(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataKi: 3.10E+3nMAssay Description:Inhibition constant against AICAR formyltransferaseMore data for this Ligand-Target Pair
TargetBifunctional purine biosynthesis protein ATIC(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataKi: 8.00E+3nMAssay Description:Inhibition constant against AICAR formyltransferaseMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0700nMAssay Description:Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.180nMAssay Description:Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.210nMAssay Description:Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.220nMAssay Description:Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.360nMAssay Description:Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.380nMAssay Description:Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.390nMAssay Description:Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.440nMAssay Description:Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.460nMpH: 7.15Assay Description:This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc...More data for this Ligand-Target Pair
Affinity DataIC50: 0.480nMAssay Description:Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Flouresence...More data for this Ligand-Target Pair
Affinity DataIC50: 0.570nMAssay Description:Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.580nMAssay Description:Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.580nMAssay Description:Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.610nMAssay Description:Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.820nMpH: 7.15Assay Description:This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Flouresence...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMpH: 7.15Assay Description:This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc...More data for this Ligand-Target Pair
Affinity DataIC50: 1.19nMAssay Description:Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMpH: 7.15Assay Description:This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMpH: 7.15Assay Description:This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc...More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMpH: 7.15Assay Description:This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc...More data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMpH: 7.15Assay Description:This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc...More data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMpH: 7.15Assay Description:This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc...More data for this Ligand-Target Pair
Affinity DataIC50: 1.80nMpH: 7.15Assay Description:This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc...More data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMpH: 7.15Assay Description:This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc...More data for this Ligand-Target Pair
Affinity DataIC50: 2.30nMpH: 7.15Assay Description:This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc...More data for this Ligand-Target Pair
Affinity DataIC50: 2.5nMpH: 7.15Assay Description:This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc...More data for this Ligand-Target Pair
Affinity DataIC50: 2.60nMpH: 7.15Assay Description:This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc...More data for this Ligand-Target Pair
Affinity DataIC50: 2.90nMpH: 7.15Assay Description:This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Flouresence...More data for this Ligand-Target Pair
Affinity DataIC50: 3.10nMpH: 7.15Assay Description:This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc...More data for this Ligand-Target Pair
Affinity DataIC50: 3.20nMpH: 7.15Assay Description:This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc...More data for this Ligand-Target Pair
Affinity DataIC50: 3.27nMAssay Description:Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 3.30nMpH: 7.15Assay Description:This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc...More data for this Ligand-Target Pair
Affinity DataIC50: 3.5nMpH: 7.15Assay Description:This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc...More data for this Ligand-Target Pair
Affinity DataIC50: 3.60nMpH: 7.15Assay Description:This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc...More data for this Ligand-Target Pair
Affinity DataIC50: 3.70nMpH: 7.15Assay Description:This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc...More data for this Ligand-Target Pair
Affinity DataIC50: 4.20nMpH: 7.15Assay Description:This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc...More data for this Ligand-Target Pair
Affinity DataIC50: 4.21nMAssay Description:This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Flouresence...More data for this Ligand-Target Pair
Affinity DataIC50: 4.21nMAssay Description:The assay is a capture of radioactive 33P phosphorylated product through filtration. The interactions of Btk, biotinylated SH2 peptide substrate (Src...More data for this Ligand-Target Pair
Affinity DataIC50: 4.21nMAssay Description:This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc...More data for this Ligand-Target Pair
Affinity DataIC50: 4.30nMpH: 7.15Assay Description:This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc...More data for this Ligand-Target Pair
Affinity DataIC50: 4.60nMpH: 7.15Assay Description:This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Flouresence...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMpH: 7.15Assay Description:This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc...More data for this Ligand-Target Pair
TargetDual specificity tyrosine-phosphorylation-regulated kinase 1A(Homo sapiens (Human))
Hoffmann-La Roche
Curated by ChEMBL
Hoffmann-La Roche
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of DYRK1A (unknown origin)More data for this Ligand-Target Pair