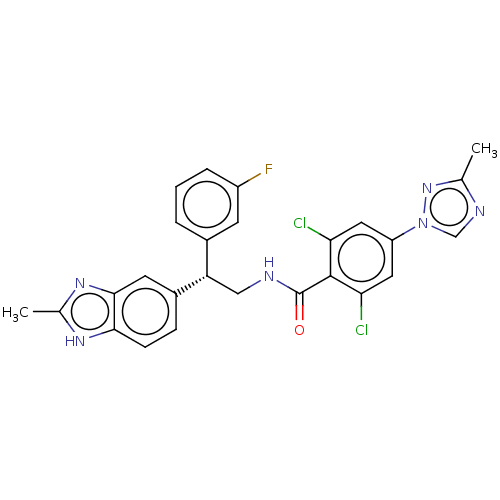
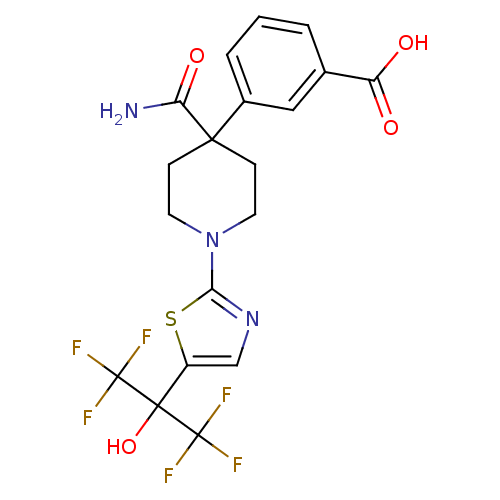
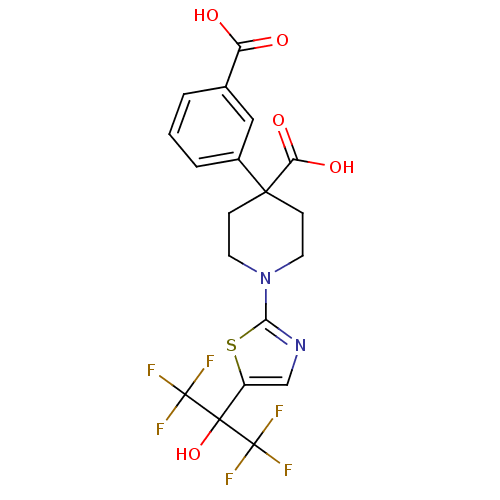
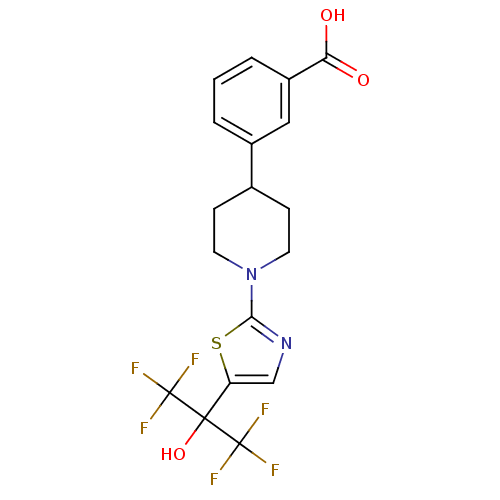
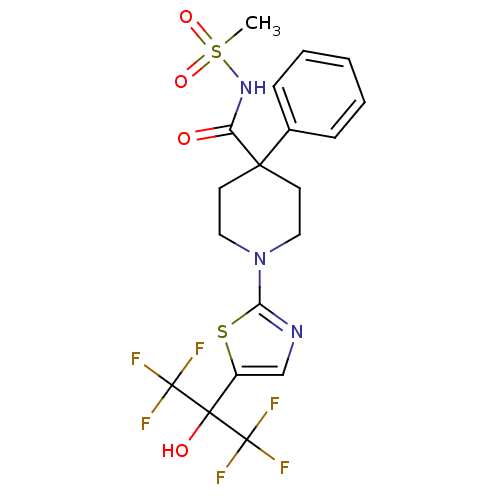
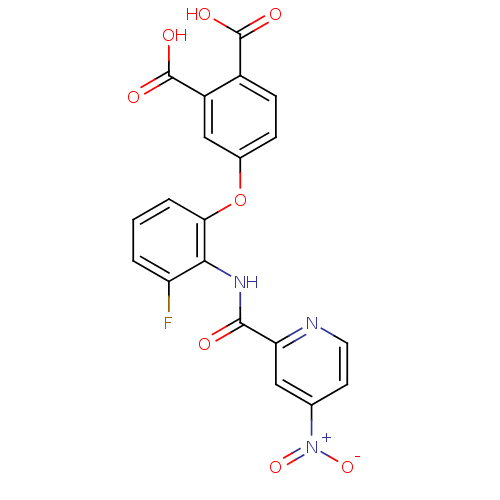
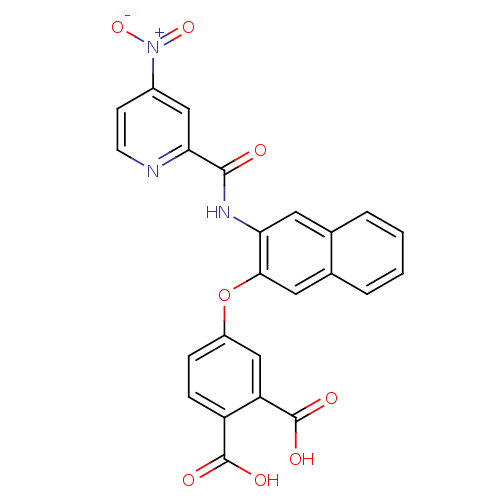
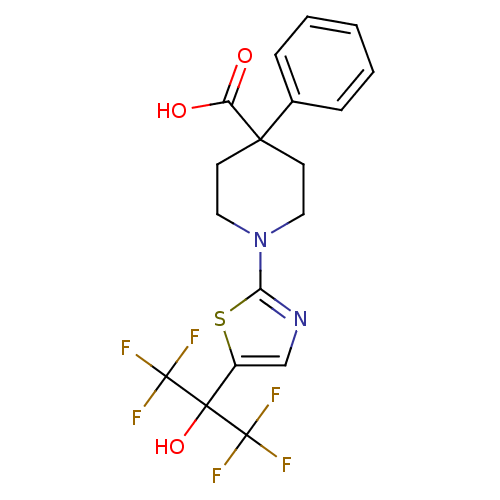
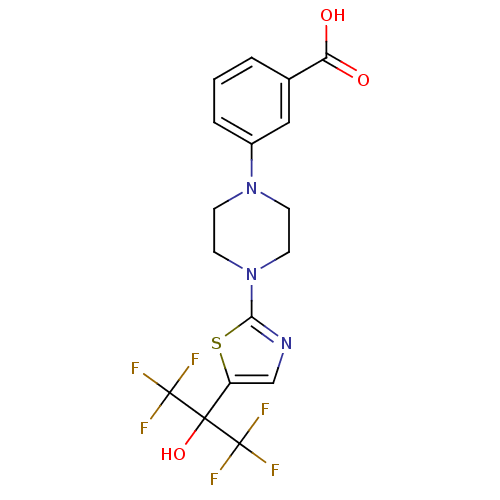
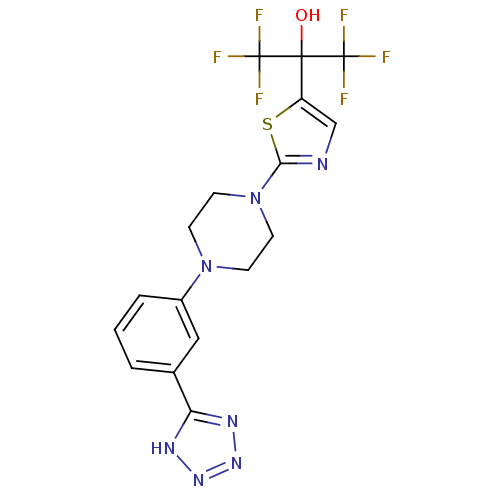
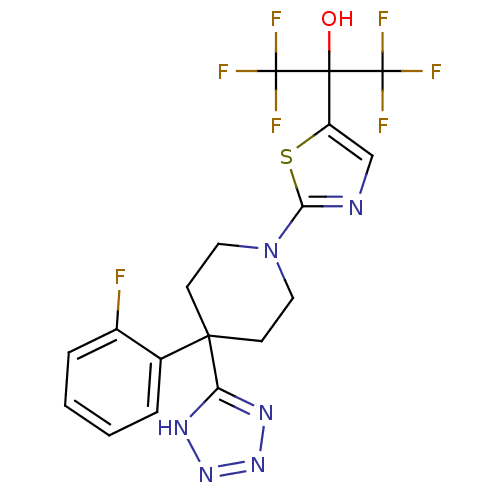
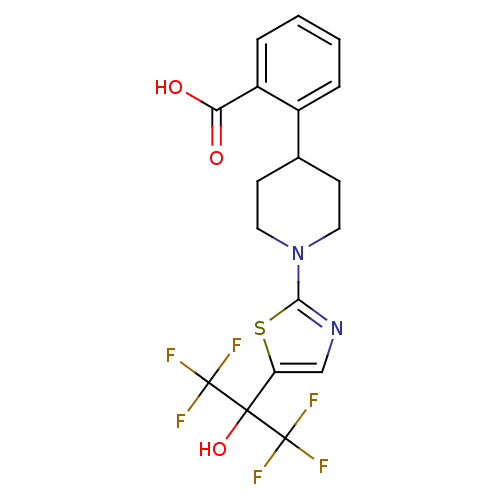
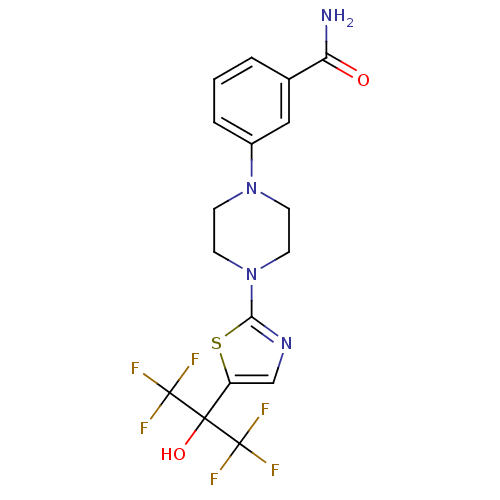
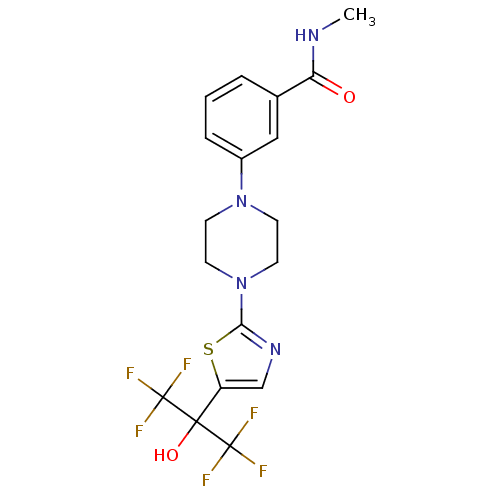
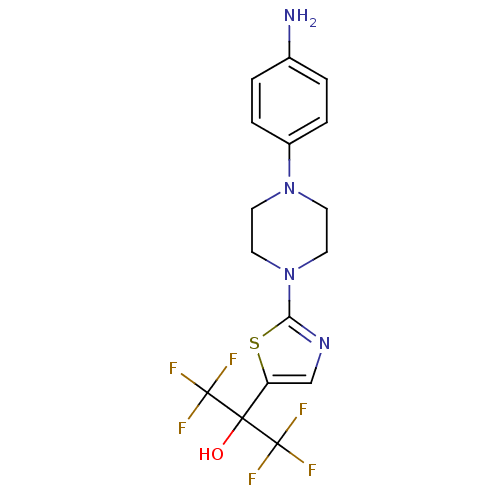
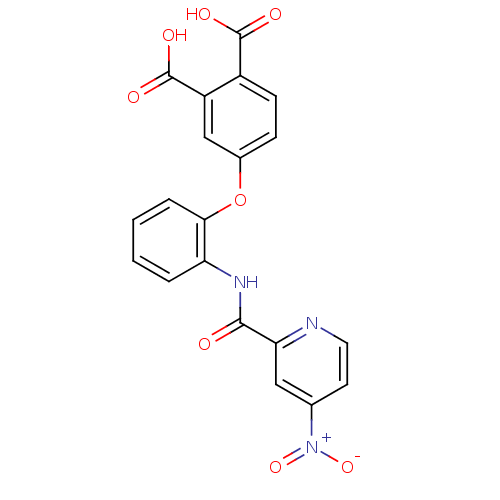
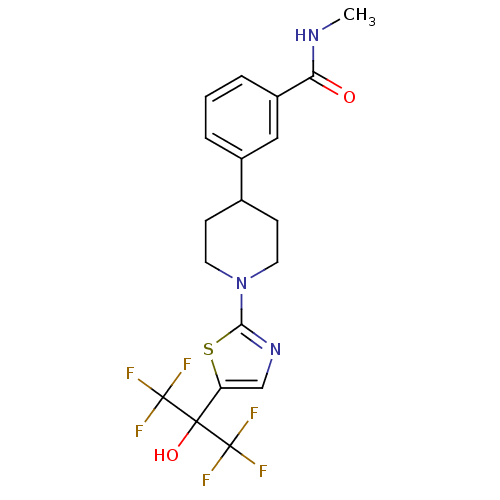
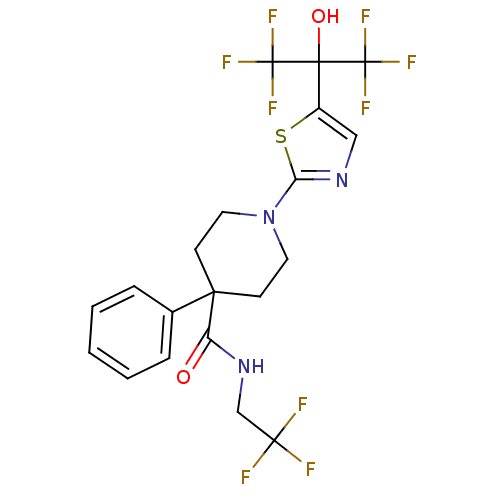
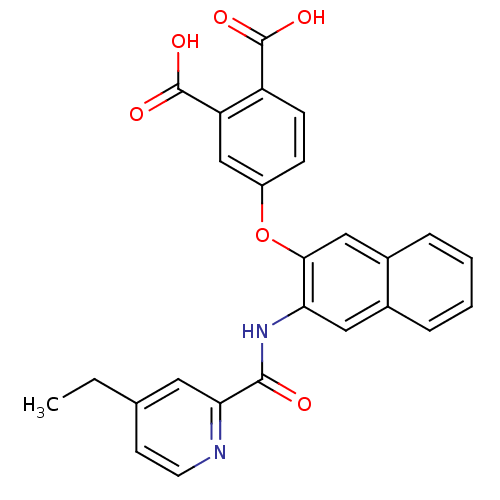
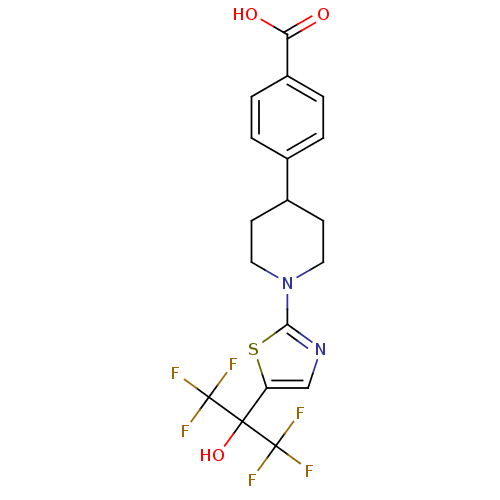
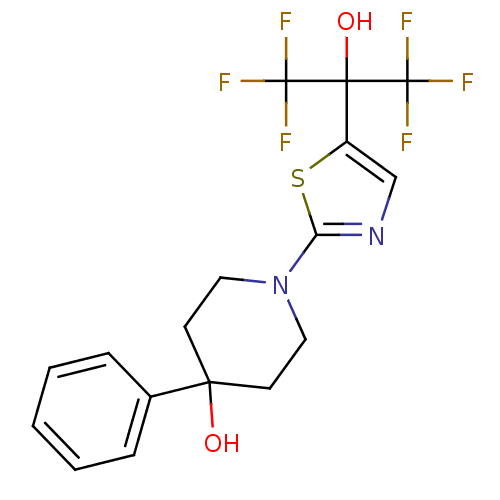
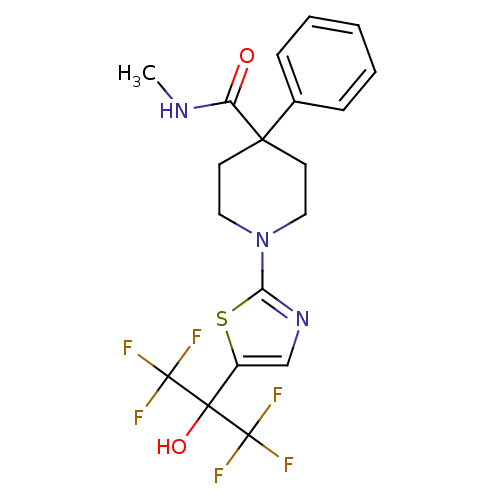
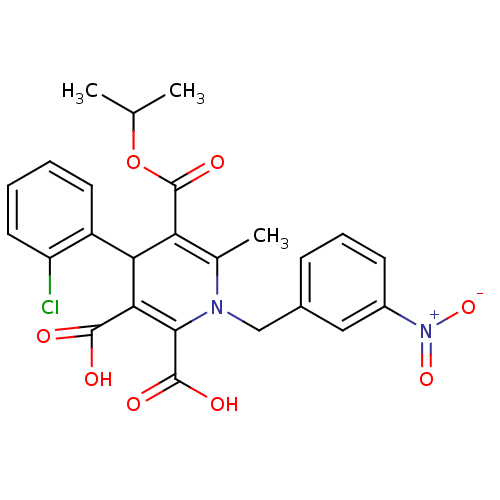
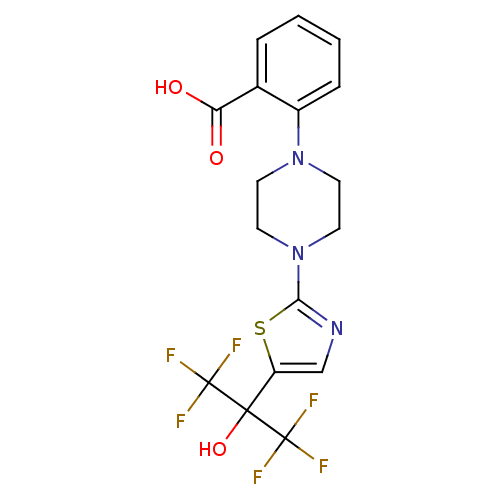
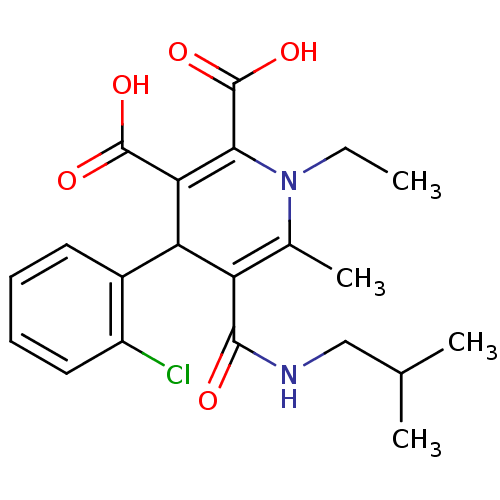
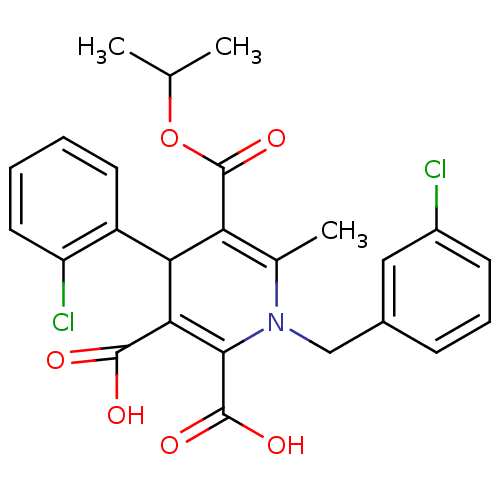
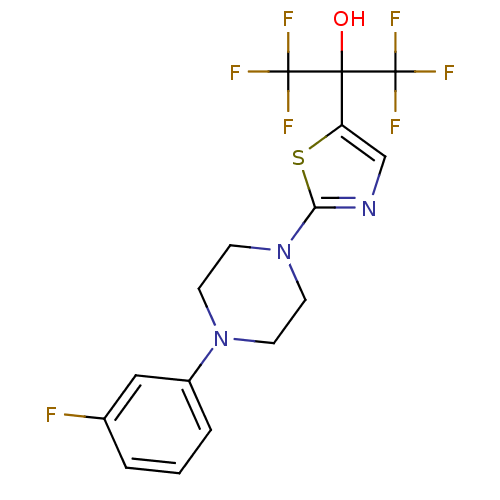
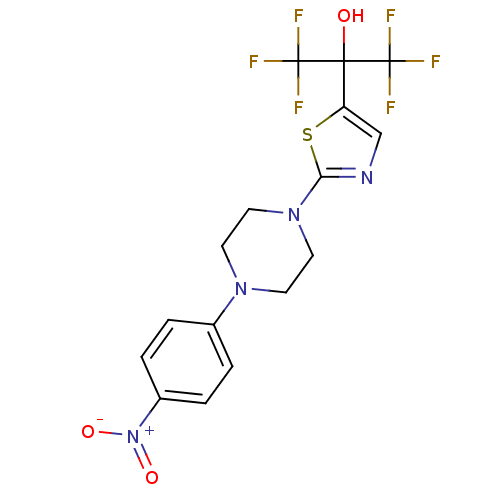
Affinity DataKi: 9.10nMAssay Description:Inhibition of human factor 9a using CH3SO2-DCHG-Gly-Arg-AFC.AcOH as substrate preinubated for 30 mins followed by substrate addition measured after 1...More data for this Ligand-Target Pair
Affinity DataKi: 11nMAssay Description:Inhibition of human factor 9a using CH3SO2-DCHG-Gly-Arg-AFC.AcOH as substrate preinubated for 30 mins followed by substrate addition measured after 1...More data for this Ligand-Target Pair
Affinity DataKi: 2.80E+3nMAssay Description:Inhibition of human factor 10a using n-Acetyl-KPR-AFC as substrate preinubated for 30 mins followed by substrate addition measured after 1 hr by fluo...More data for this Ligand-Target Pair
TargetMalonyl-CoA decarboxylase, mitochondrial(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Inhibition of human MCDMore data for this Ligand-Target Pair
TargetMalonyl-CoA decarboxylase, mitochondrial(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.600nMAssay Description:Inhibition of human MCDMore data for this Ligand-Target Pair
TargetMalonyl-CoA decarboxylase, mitochondrial(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.900nMAssay Description:Inhibition of human MCDMore data for this Ligand-Target Pair
TargetMalonyl-CoA decarboxylase, mitochondrial(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.900nMAssay Description:Inhibition of human MCDMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibitory activity against HLGP(human liver glycogen phosphorylase)More data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibitory activity against HLGP(human liver glycogen phosphorylase)More data for this Ligand-Target Pair
TargetMalonyl-CoA decarboxylase, mitochondrial(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.10nMAssay Description:Inhibition of human MCDMore data for this Ligand-Target Pair
TargetMalonyl-CoA decarboxylase, mitochondrial(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.30nMAssay Description:Inhibition of human MCDMore data for this Ligand-Target Pair
TargetMalonyl-CoA decarboxylase, mitochondrial(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.40nMAssay Description:Inhibition of human MCDMore data for this Ligand-Target Pair
TargetMalonyl-CoA decarboxylase, mitochondrial(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.60nMAssay Description:Inhibition of human MCDMore data for this Ligand-Target Pair
TargetMalonyl-CoA decarboxylase, mitochondrial(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.60nMAssay Description:Inhibition of human MCDMore data for this Ligand-Target Pair
TargetMalonyl-CoA decarboxylase, mitochondrial(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.70nMAssay Description:Inhibition of human MCDMore data for this Ligand-Target Pair
TargetMalonyl-CoA decarboxylase, mitochondrial(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.80nMAssay Description:Inhibition of human MCDMore data for this Ligand-Target Pair
Affinity DataIC50: 1.90nMAssay Description:Inhibition of human factor 9a using CH3SO2-DCHG-Gly-Arg-AFC.AcOH as substrate preinubated for 30 mins followed by substrate addition measured after 1...More data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibitory activity against HLGP(human liver glycogen phosphorylase)More data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibitory activity against HLGP(human liver glycogen phosphorylase)More data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:In vitro inhibitory activity against human liver glycogen phosphorylase a (HLGPa)More data for this Ligand-Target Pair
TargetMalonyl-CoA decarboxylase, mitochondrial(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 2.20nMAssay Description:Inhibition of human MCDMore data for this Ligand-Target Pair
TargetMalonyl-CoA decarboxylase, mitochondrial(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 2.30nMAssay Description:Inhibition of human MCDMore data for this Ligand-Target Pair
TargetMalonyl-CoA decarboxylase, mitochondrial(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 2.70nMAssay Description:Inhibition of human MCDMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibitory activity against HLGP(human liver glycogen phosphorylase)More data for this Ligand-Target Pair
TargetGlycogen phosphorylase, muscle form(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibitory activity against HMGP(human muscle glycogen phosphorylase)More data for this Ligand-Target Pair
TargetMalonyl-CoA decarboxylase, mitochondrial(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 3.10nMAssay Description:Inhibition of human MCDMore data for this Ligand-Target Pair
TargetMalonyl-CoA decarboxylase, mitochondrial(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 3.20nMAssay Description:Inhibition of human MCDMore data for this Ligand-Target Pair
TargetMalonyl-CoA decarboxylase, mitochondrial(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 3.30nMAssay Description:Inhibition of human MCDMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:In vitro inhibitory activity against human liver glycogen phosphorylase a (HLGPa)More data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibitory activity against HLGP(human liver glycogen phosphorylase)More data for this Ligand-Target Pair
TargetMalonyl-CoA decarboxylase, mitochondrial(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of human MCDMore data for this Ligand-Target Pair
TargetMalonyl-CoA decarboxylase, mitochondrial(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 4.5nMAssay Description:Inhibition of human MCDMore data for this Ligand-Target Pair
TargetMalonyl-CoA decarboxylase, mitochondrial(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 4.70nMAssay Description:Inhibition of human MCDMore data for this Ligand-Target Pair
TargetMalonyl-CoA decarboxylase, mitochondrial(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 4.90nMAssay Description:Inhibition of human MCDMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:In vitro inhibitory activity against human liver glycogen phosphorylase a (HLGPa)More data for this Ligand-Target Pair
TargetMalonyl-CoA decarboxylase, mitochondrial(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 5.10nMAssay Description:Inhibition of human MCDMore data for this Ligand-Target Pair
TargetMalonyl-CoA decarboxylase, mitochondrial(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 5.70nMAssay Description:Inhibition of human MCDMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, muscle form(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:In vitro inhibitory activity against human muscle glycogen phosphorylase a (HMGPa)More data for this Ligand-Target Pair
TargetMalonyl-CoA decarboxylase, mitochondrial(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 6.70nMAssay Description:Inhibition of human MCDMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 7.88nMAssay Description:In vitro inhibitory activity against human liver glycogen phosphorylase a (HLGPa)More data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 8nMAssay Description:In vitro inhibitory activity against human liver glycogen phosphorylase a (HLGPa)More data for this Ligand-Target Pair
TargetMalonyl-CoA decarboxylase, mitochondrial(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 8.10nMAssay Description:Inhibition of human MCDMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, muscle form(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 8.28nMAssay Description:In vitro inhibitory activity against human muscle glycogen phosphorylase a (HMGPa)More data for this Ligand-Target Pair
TargetMalonyl-CoA decarboxylase, mitochondrial(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 8.70nMAssay Description:Inhibition of human MCDMore data for this Ligand-Target Pair
TargetMalonyl-CoA decarboxylase, mitochondrial(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 8.90nMAssay Description:Inhibition of human MCDMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, muscle form(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 9nMAssay Description:Inhibitory activity against HMGP(human muscle glycogen phosphorylase)More data for this Ligand-Target Pair
Affinity DataIC50: <9nMAssay Description:Inhibitory activity against mouse liver glycogen phosphorylaseMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Rattus norvegicus)
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: <9nMAssay Description:Inhibitory activity against rat liver glycogen phosphorylaseMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 9.10nMAssay Description:In vitro inhibitory activity against human liver glycogen phosphorylase a (HLGPa)More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of human factor 9a using CH3SO2-DCHG-Gly-Arg-AFC.AcOH as substrate preinubated for 30 mins followed by substrate addition measured after 1...More data for this Ligand-Target Pair