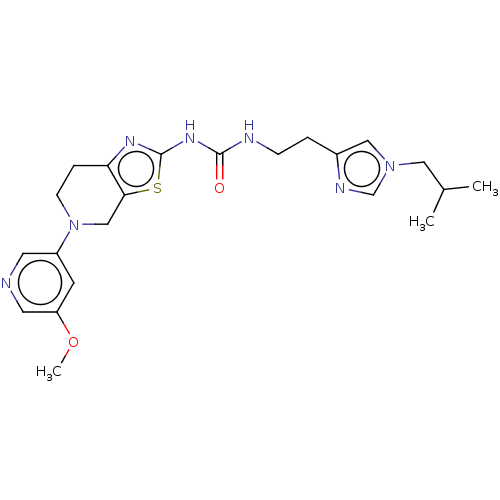
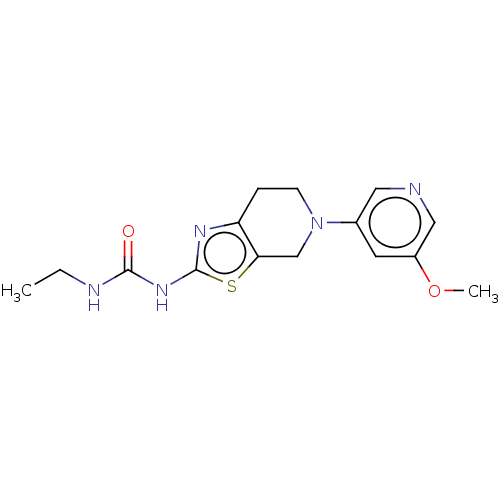
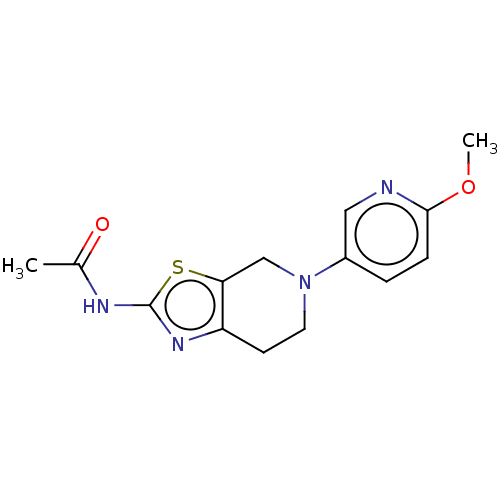
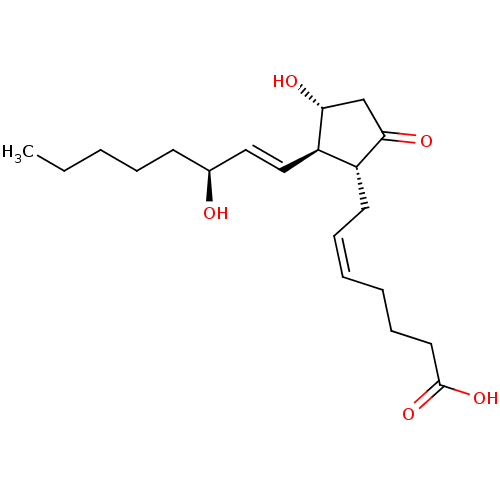
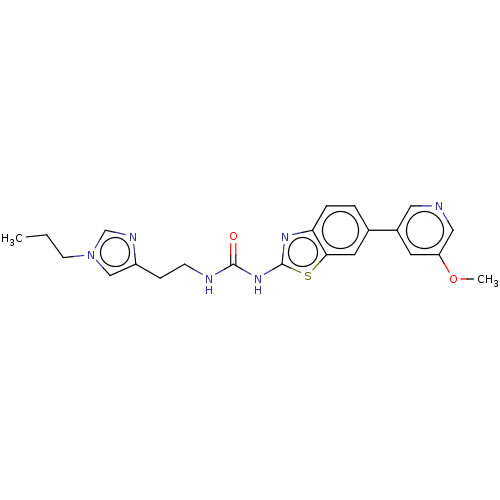
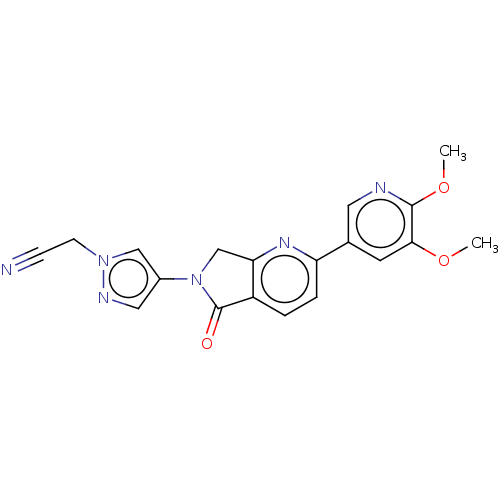
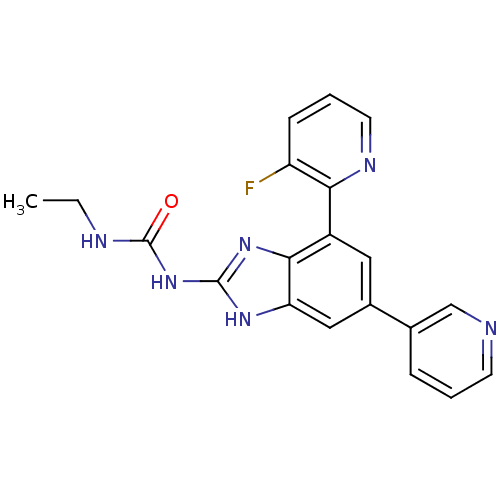
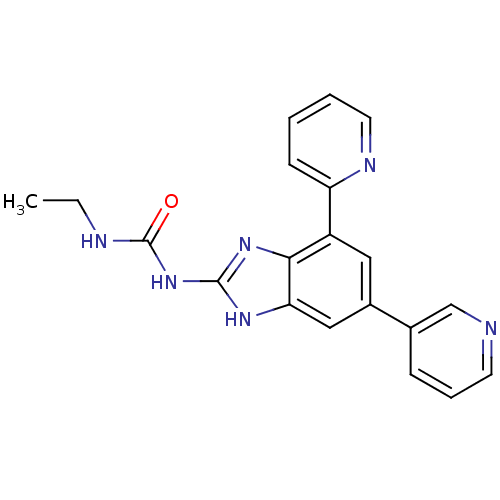
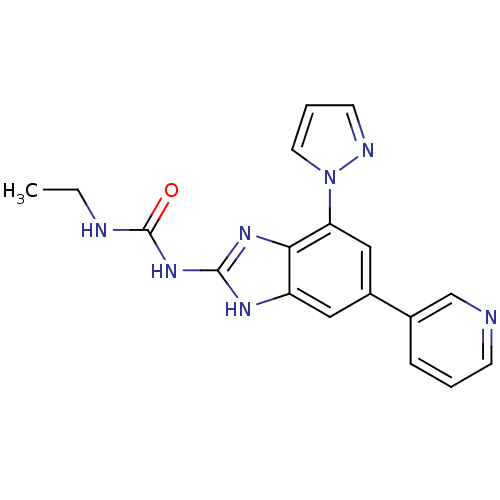
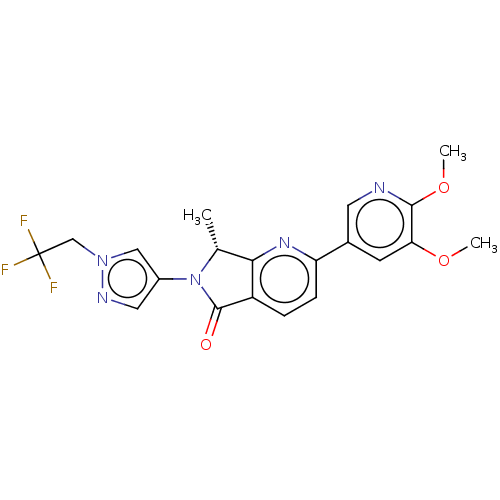
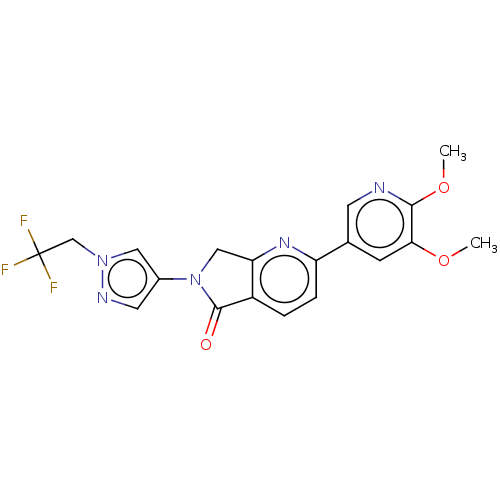
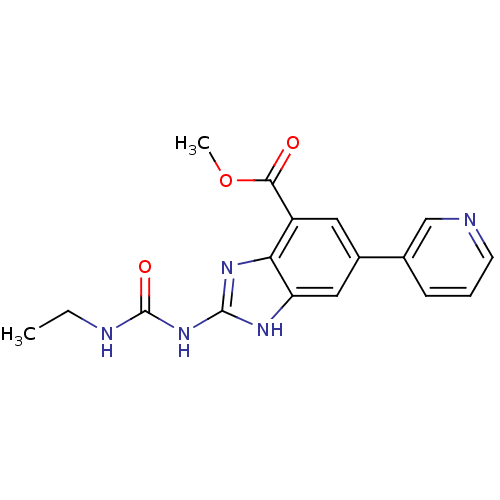
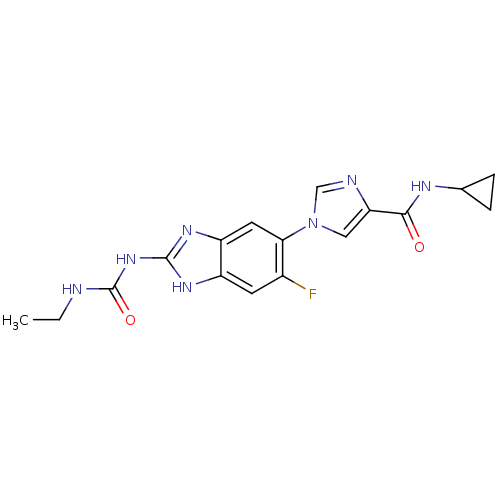
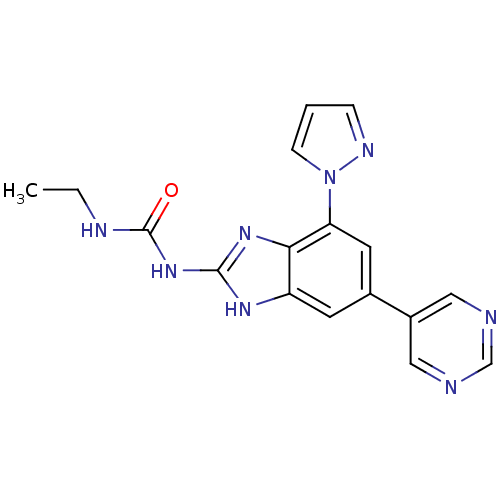
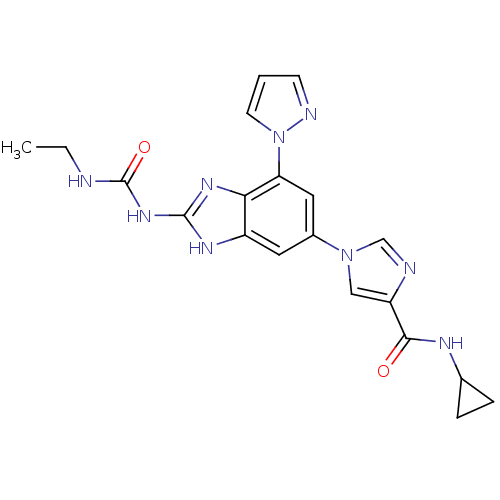
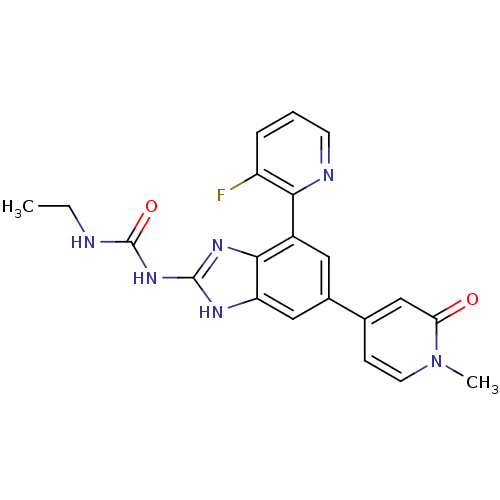
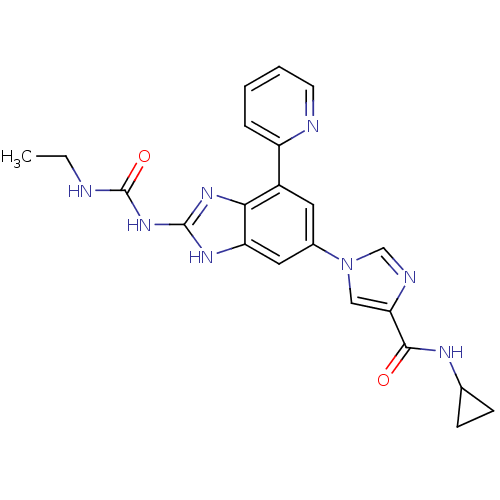
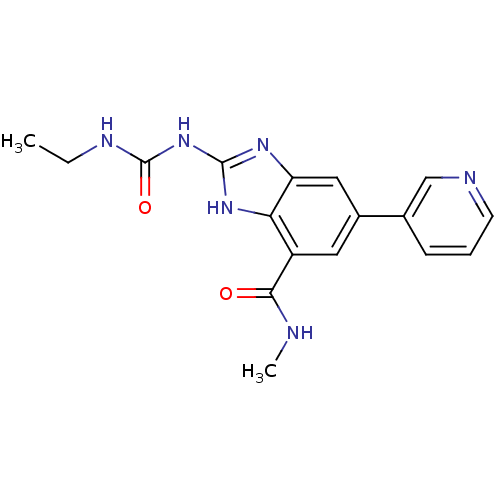
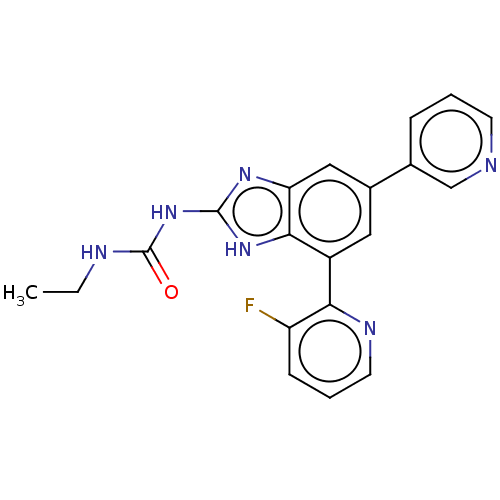
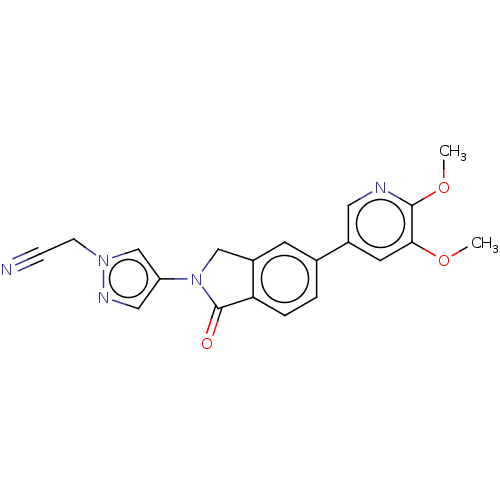
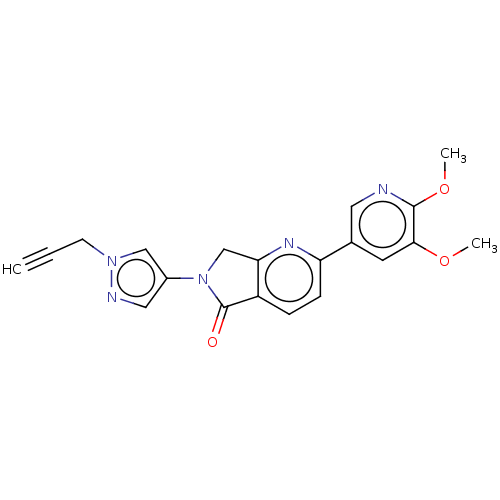
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 0.00200nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
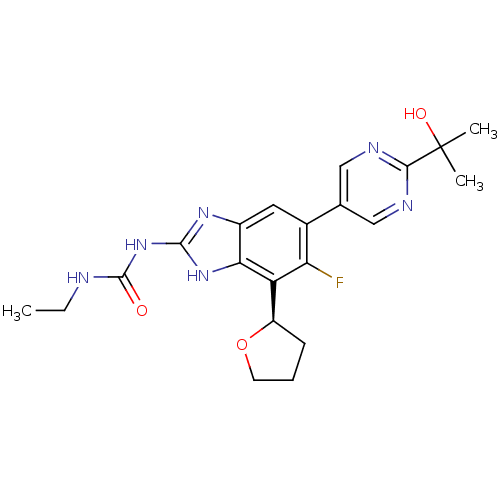
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 0.00300nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 0.00300nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 0.00600nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 0.00900nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 0.0100nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 0.0110nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 0.0130nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 0.0160nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 0.0170nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 0.0240nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 0.0250nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 0.0340nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 0.0480nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 0.0660nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 0.157nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 0.330nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP4 subtype(Homo sapiens (Human))
Emd-Serono Research Institute
Curated by ChEMBL
Emd-Serono Research Institute
Curated by ChEMBL
Affinity DataKi: 0.790nMAssay Description:Binding affinity at human prostaglandin EP4 receptorMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 3nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 3.10nMAssay Description:Inhibition of N-terminal His6-tagged SARS-CoV2 Wuhan-Hu-1 main protease expressed in Escherichia coli using Dabcyl-KTSAVLQSGFRKME-Edans as substrate ...More data for this Ligand-Target Pair
Affinity DataKi: 3.80nMAssay Description:Binding affinity which represents concentration giving half-maximal inhibition of [3H]spiperone (Dopamine receptor D2) binding to rat tissue homogena...More data for this Ligand-Target Pair
Affinity DataKi: <4nMAssay Description:Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ...More data for this Ligand-Target Pair
Affinity DataKi: <4nMAssay Description:Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ...More data for this Ligand-Target Pair
Affinity DataKi: <4nMAssay Description:Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 4nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 4nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: <4nMAssay Description:Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ...More data for this Ligand-Target Pair
Affinity DataKi: <4nMAssay Description:Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ...More data for this Ligand-Target Pair
Affinity DataKi: <4nMAssay Description:Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ...More data for this Ligand-Target Pair
Affinity DataKi: <4nMAssay Description:Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ...More data for this Ligand-Target Pair
Affinity DataKi: <4nMAssay Description:Inhibition of aureus Escherichia coli DNA gyrase A2B2 using pBR322 plasmid DNA as substrate by coupled enzyme reaction assayMore data for this Ligand-Target Pair
Affinity DataKi: <4nMAssay Description:Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ...More data for this Ligand-Target Pair
Affinity DataKi: <4nMAssay Description:Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ...More data for this Ligand-Target Pair
Affinity DataKi: 4.10nMAssay Description:Binding affinity which represents concentration giving half-maximal inhibition of [3H]7-OH-DPAT binding to Dopamine receptor D3 in rat tissue homogen...More data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP2 subtype(Homo sapiens (Human))
Emd-Serono Research Institute
Curated by ChEMBL
Emd-Serono Research Institute
Curated by ChEMBL
Affinity DataKi: 4.90nMAssay Description:Binding affinity at human prostaglandin EP2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Inhibition of aureus Escherichia coli DNA gyrase A2B2 using pBR322 plasmid DNA as substrate by coupled enzyme reaction assayMore data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ...More data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:Inhibition of aureus Escherichia coli DNA gyrase A2B2 using pBR322 plasmid DNA as substrate by coupled enzyme reaction assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 6nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ...More data for this Ligand-Target Pair
Affinity DataKi: 7nMAssay Description:Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ...More data for this Ligand-Target Pair
Affinity DataKi: 7nM ΔG°: -47.3kJ/molepH: 7.5 T: 2°CAssay Description:Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 7nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 8nM ΔG°: -47.0kJ/molepH: 7.5 T: 2°CAssay Description:Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 8nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 9nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 9nMAssay Description:Inhibition of Staphylococcus aureus DNA gyrase using pBR322 plasmid DNA as substrate by coupled enzyme reaction assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 9nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 10nM ΔG°: -46.4kJ/molepH: 7.5 T: 2°CAssay Description:Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ...More data for this Ligand-Target Pair

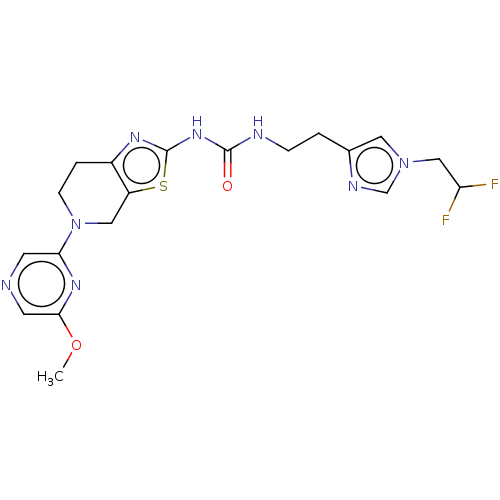
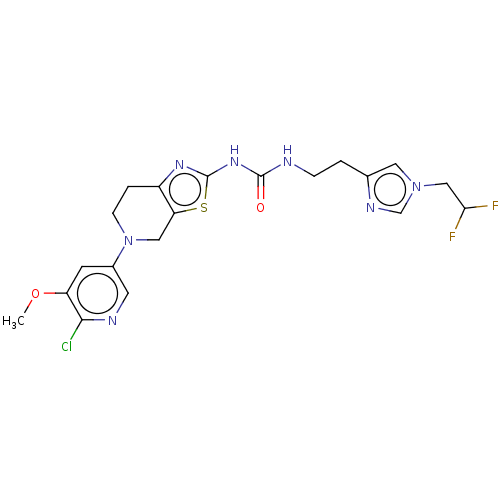

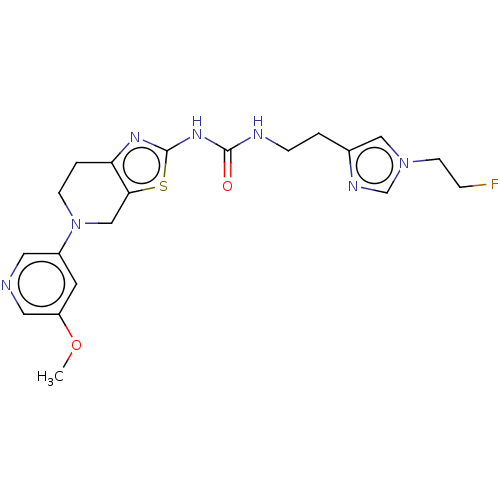


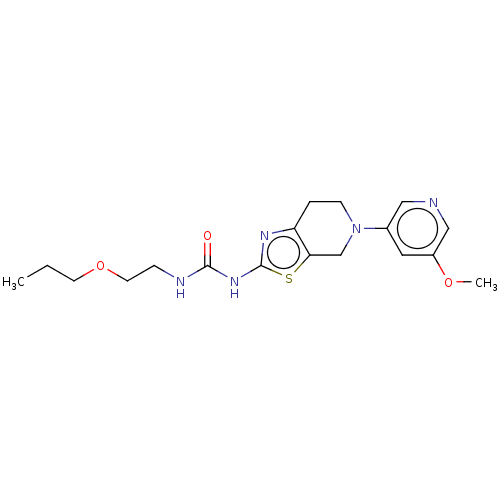
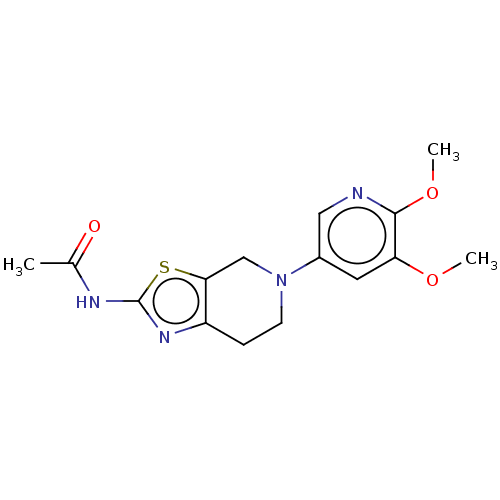
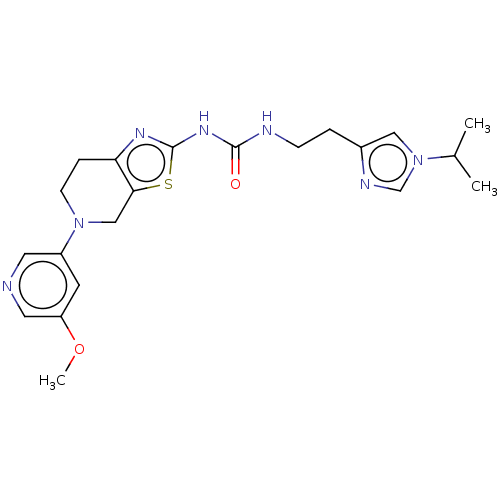
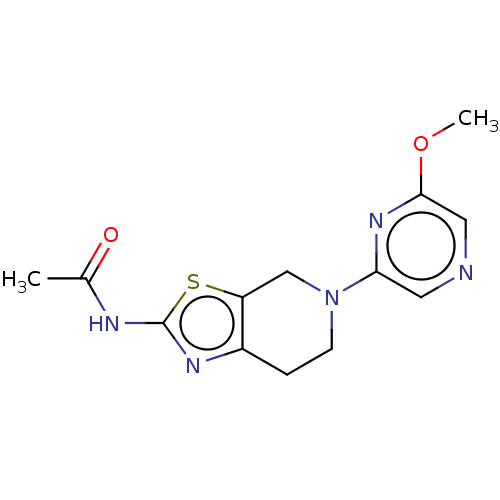
 3D Structure (crystal)
3D Structure (crystal)