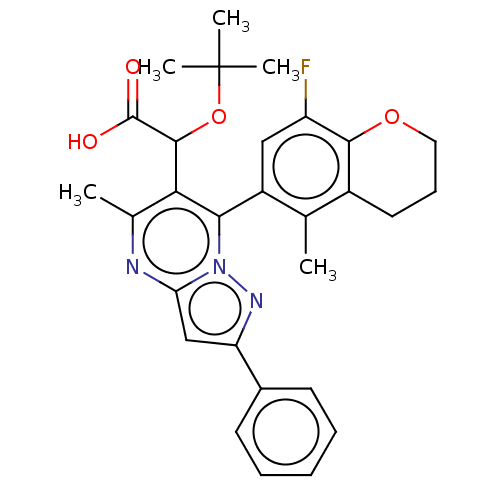
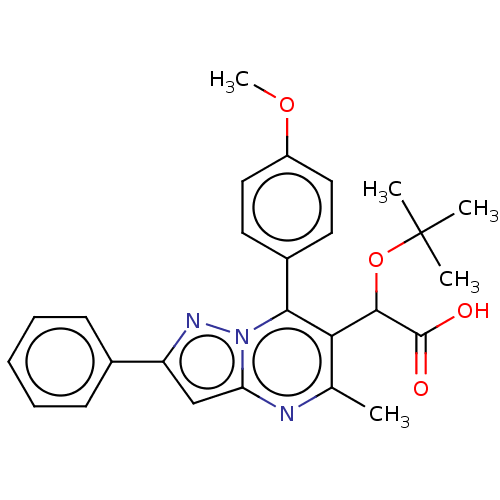
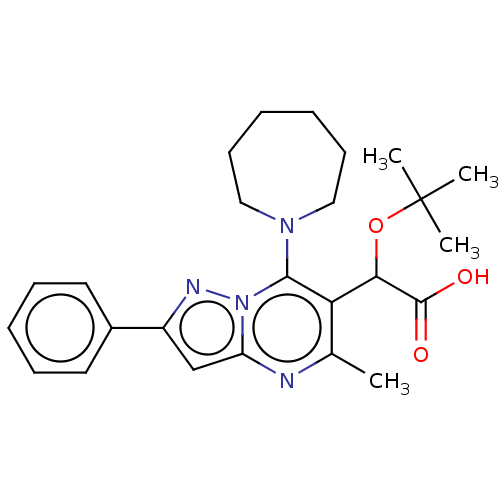
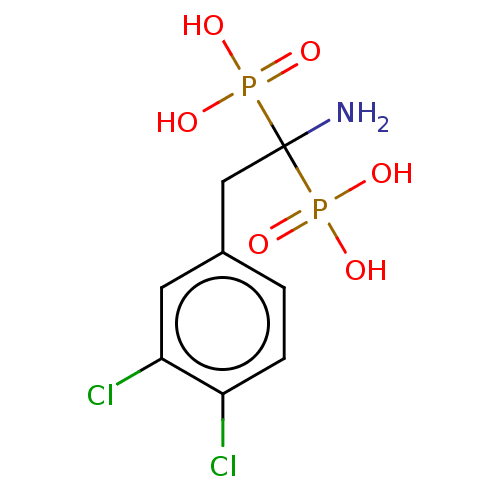
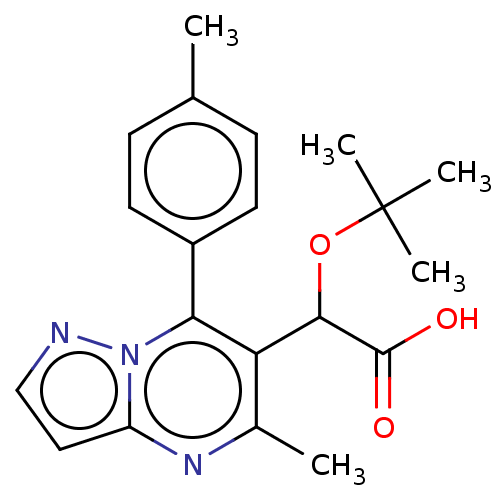
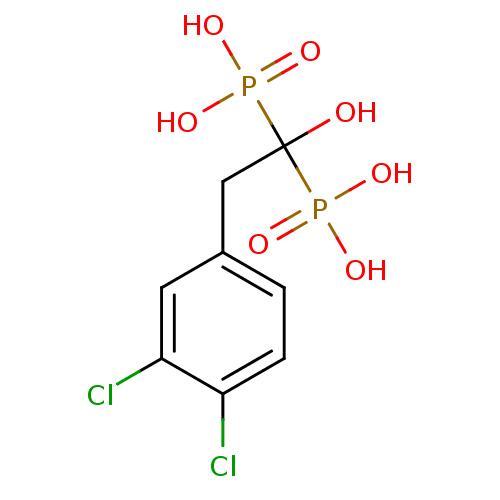
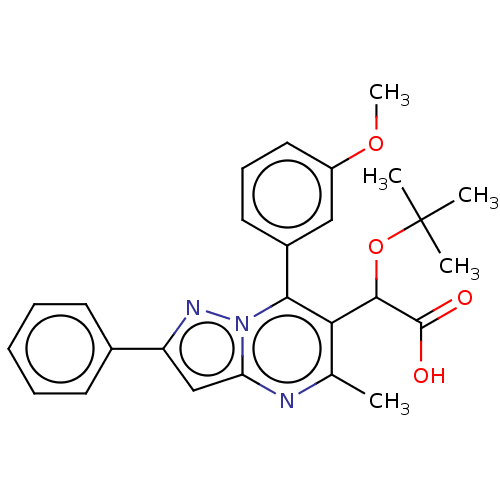
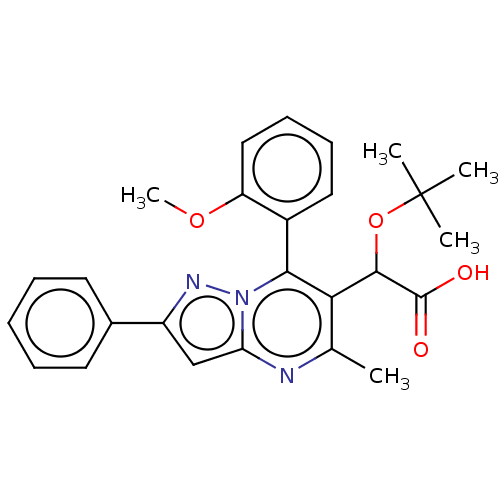
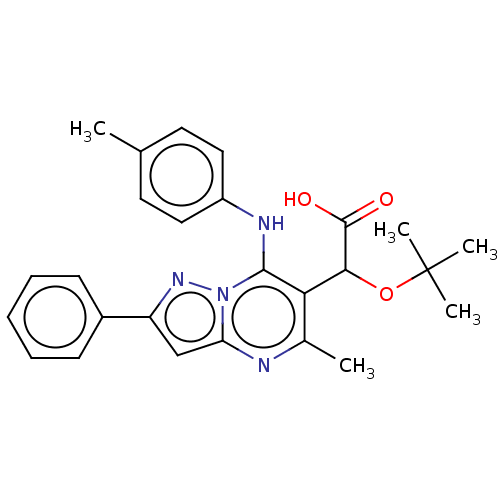
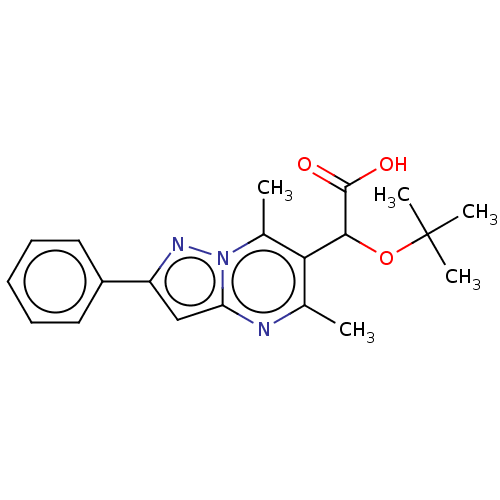
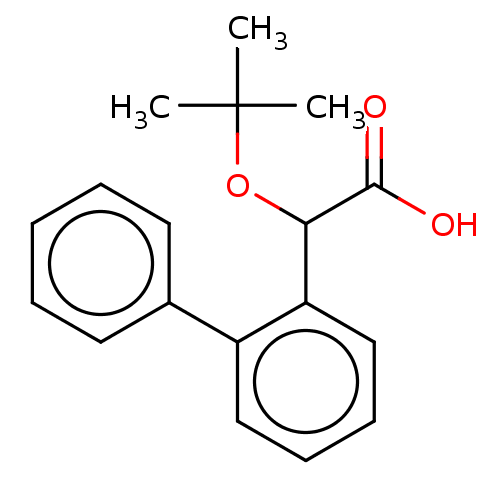
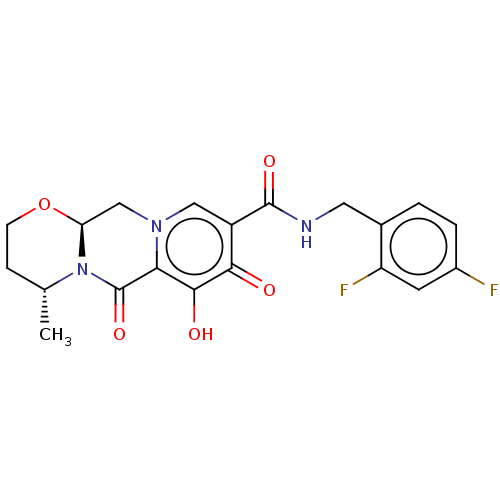
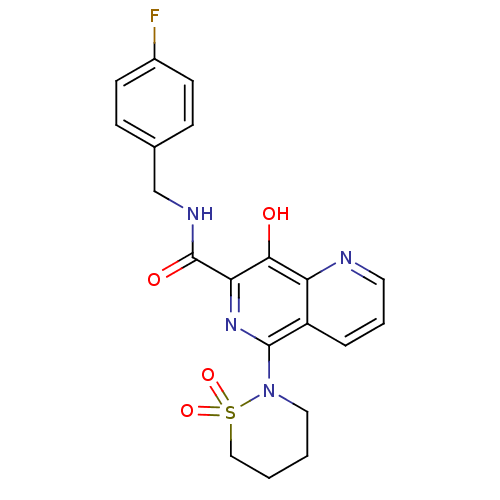
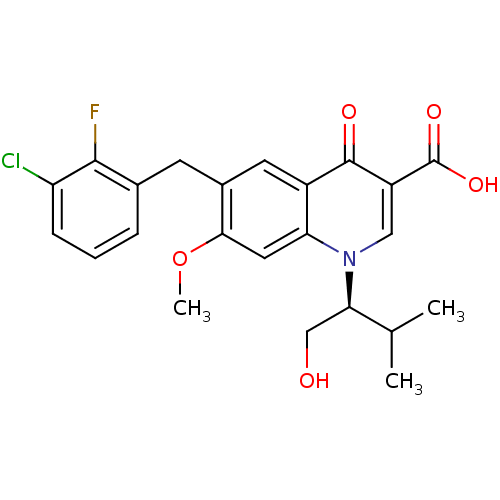
Affinity DataKi: 8nMAssay Description:Binding affinity to wild type HIV1 integrase G140S/Q148H mutantMore data for this Ligand-Target Pair
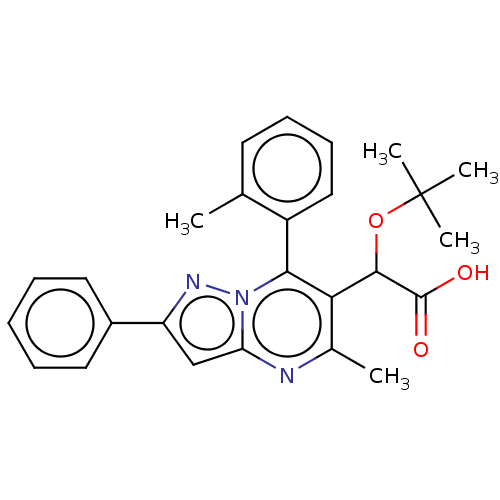
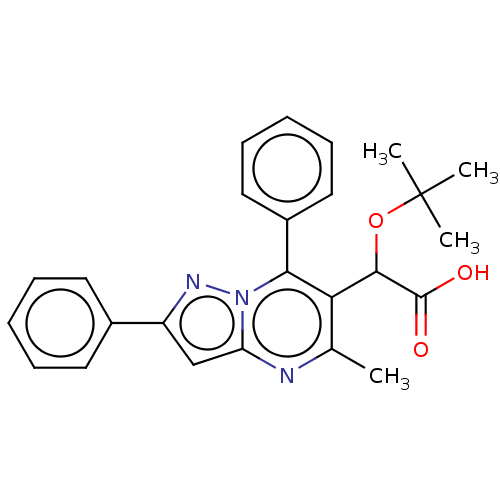
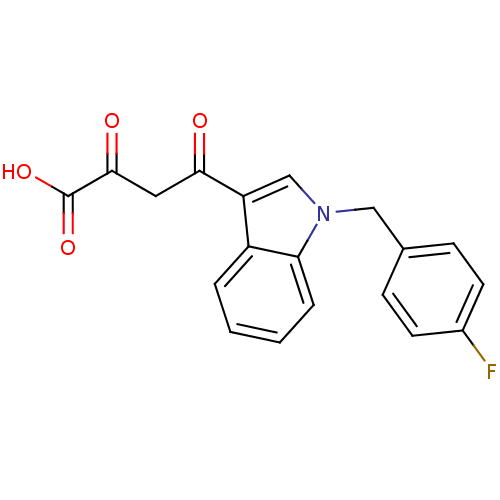
Affinity DataKi: 17nMAssay Description:Binding affinity to wild type HIV1 integrase G140S/Q148H mutantMore data for this Ligand-Target Pair
TargetIntegrase(Human immunodeficiency virus 1)
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataKi: 40nMAssay Description:Allosteric inhibition of [3H]-2-tert-butoxy-2-[2-methyl-4-(p-tolyl)-3-quinolyl]acetic acid binding to HIV1 integrase measured after 1 hr under dark c...More data for this Ligand-Target Pair
TargetIntegrase(Human immunodeficiency virus 1)
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataKi: 60nMAssay Description:Allosteric inhibition of [3H]-2-tert-butoxy-2-[2-methyl-4-(p-tolyl)-3-quinolyl]acetic acid binding to HIV1 integrase measured after 1 hr under dark c...More data for this Ligand-Target Pair
TargetIntegrase(Human immunodeficiency virus 1)
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataKi: 90nMAssay Description:Allosteric inhibition of [3H]-2-tert-butoxy-2-[2-methyl-4-(p-tolyl)-3-quinolyl]acetic acid binding to HIV1 integrase measured after 1 hr under dark c...More data for this Ligand-Target Pair
Affinity DataKi: 130nMAssay Description:Binding affinity to influenza A/Beijing/262/95 H1N1 neuraminidase expressed in baculovirus expression system using sialyl-lactose as substrateMore data for this Ligand-Target Pair
TargetIntegrase(Human immunodeficiency virus 1)
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataKi: 170nMAssay Description:Allosteric inhibition of [3H]-2-tert-butoxy-2-[2-methyl-4-(p-tolyl)-3-quinolyl]acetic acid binding to HIV1 integrase measured after 1 hr under dark c...More data for this Ligand-Target Pair
TargetIntegrase(Human immunodeficiency virus 1)
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataKi: 170nMAssay Description:Allosteric inhibition of [3H]-2-tert-butoxy-2-[2-methyl-4-(p-tolyl)-3-quinolyl]acetic acid binding to HIV1 integrase measured after 1 hr under dark c...More data for this Ligand-Target Pair
Affinity DataKi: 280nMAssay Description:Binding affinity to influenza A/Beijing/262/95 H1N1 neuraminidase expressed in baculovirus expression system using sialyl-lactose as substrateMore data for this Ligand-Target Pair
TargetIntegrase(Human immunodeficiency virus 1)
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataKi: 310nMAssay Description:Allosteric inhibition of [3H]-2-tert-butoxy-2-[2-methyl-4-(p-tolyl)-3-quinolyl]acetic acid binding to HIV1 integrase measured after 1 hr under dark c...More data for this Ligand-Target Pair
TargetIntegrase(Human immunodeficiency virus 1)
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataKi: 430nMAssay Description:Allosteric inhibition of [3H]-2-tert-butoxy-2-[2-methyl-4-(p-tolyl)-3-quinolyl]acetic acid binding to HIV1 integrase measured after 1 hr under dark c...More data for this Ligand-Target Pair
TargetIntegrase(Human immunodeficiency virus 1)
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataKi: 680nMAssay Description:Allosteric inhibition of [3H]-2-tert-butoxy-2-[2-methyl-4-(p-tolyl)-3-quinolyl]acetic acid binding to HIV1 integrase measured after 1 hr under dark c...More data for this Ligand-Target Pair
TargetIntegrase(Human immunodeficiency virus 1)
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataKi: 1.00E+3nMAssay Description:Non-competitive inhibition of HIV-1 integrase expressed in Escherichia coli using [32P]-labeled U5B/U5A DNA as substrate after 1 hr by double-recipro...More data for this Ligand-Target Pair
TargetIntegrase(Human immunodeficiency virus 1)
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataKi: 2.00E+3nMAssay Description:Non-competitive inhibition of HIV-1 integrase expressed in Escherichia coli using [32P]-labeled U5B/U5A DNA as substrate after 1 hr by double-recipro...More data for this Ligand-Target Pair
TargetIntegrase(Human immunodeficiency virus 1)
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataKi: 2.00E+3nMAssay Description:Allosteric inhibition of [3H]-2-tert-butoxy-2-[2-methyl-4-(p-tolyl)-3-quinolyl]acetic acid binding to HIV1 integrase measured after 1 hr under dark c...More data for this Ligand-Target Pair
Affinity DataKi: 2.90E+3nMAssay Description:Binding affinity to influenza A/Beijing/262/95 H1N1 neuraminidase expressed in baculovirus expression system using sialyl-lactose as substrateMore data for this Ligand-Target Pair
TargetIntegrase(Human immunodeficiency virus 1)
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataKi: 3.40E+3nMAssay Description:Allosteric inhibition of [3H]-2-tert-butoxy-2-[2-methyl-4-(p-tolyl)-3-quinolyl]acetic acid binding to HIV1 integrase measured after 1 hr under dark c...More data for this Ligand-Target Pair
TargetIntegrase(Human immunodeficiency virus 1)
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataKi: 3.70E+3nMAssay Description:Allosteric inhibition of [3H]-2-tert-butoxy-2-[2-methyl-4-(p-tolyl)-3-quinolyl]acetic acid binding to HIV1 integrase measured after 1 hr under dark c...More data for this Ligand-Target Pair
TargetIntegrase(Human immunodeficiency virus 1)
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataKi: 4.40E+3nMAssay Description:Non-competitive inhibition of HIV-1 integrase expressed in Escherichia coli using [32P]-labeled U5B/U5A DNA as substrate after 1 hr by double-recipro...More data for this Ligand-Target Pair
TargetIntegrase(Human immunodeficiency virus 1)
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataKi: 4.40E+3nMAssay Description:Allosteric inhibition of [3H]-2-tert-butoxy-2-[2-methyl-4-(p-tolyl)-3-quinolyl]acetic acid binding to HIV1 integrase measured after 1 hr under dark c...More data for this Ligand-Target Pair
TargetIntegrase(Human immunodeficiency virus 1)
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataKi: 1.53E+4nMAssay Description:Allosteric inhibition of [3H]-2-tert-butoxy-2-[2-methyl-4-(p-tolyl)-3-quinolyl]acetic acid binding to HIV1 integrase measured after 1 hr under dark c...More data for this Ligand-Target Pair
TargetIntegrase(Human immunodeficiency virus 1)
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataKi: 3.80E+4nMAssay Description:Competitive inhibition of HIV-1 integrase expressed in Escherichia coli using [32P]-labeled U5B/U5A DNA as substrate after 1 hr by double-reciprocal ...More data for this Ligand-Target Pair
TargetIntegrase(Human immunodeficiency virus 1)
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataKi: >4.00E+4nMAssay Description:Allosteric inhibition of [3H]-2-tert-butoxy-2-[2-methyl-4-(p-tolyl)-3-quinolyl]acetic acid binding to HIV1 integrase measured after 1 hr under dark c...More data for this Ligand-Target Pair
TargetIntegrase(Human immunodeficiency virus 1)
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataKi: >4.00E+4nMAssay Description:Allosteric inhibition of [3H]-2-tert-butoxy-2-[2-methyl-4-(p-tolyl)-3-quinolyl]acetic acid binding to HIV1 integrase measured after 1 hr under dark c...More data for this Ligand-Target Pair
TargetIntegrase(Human immunodeficiency virus 1)
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataKi: >4.00E+4nMAssay Description:Allosteric inhibition of [3H]-2-tert-butoxy-2-[2-methyl-4-(p-tolyl)-3-quinolyl]acetic acid binding to HIV1 integrase measured after 1 hr under dark c...More data for this Ligand-Target Pair
TargetIntegrase(Human immunodeficiency virus 1)
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataKi: >5.30E+4nMAssay Description:Allosteric inhibition of [3H]-2-tert-butoxy-2-[2-methyl-4-(p-tolyl)-3-quinolyl]acetic acid binding to HIV1 integrase measured after 1 hr under dark c...More data for this Ligand-Target Pair
TargetIntegrase(Human immunodeficiency virus 1)
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataKi: 6.70E+4nMAssay Description:Allosteric inhibition of [3H]-2-tert-butoxy-2-[2-methyl-4-(p-tolyl)-3-quinolyl]acetic acid binding to HIV1 integrase measured after 1 hr under dark c...More data for this Ligand-Target Pair
TargetIntegrase(Human immunodeficiency virus 1)
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Inhibition of HIV integrase strand transfer activityMore data for this Ligand-Target Pair
TargetIntegrase(Human immunodeficiency virus 1)
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Inhibition of HIV1 integrase using labelled oligonucleotide substrate by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:In vitro concentration required to inhibit the overall HIV-1 integrase strand transferMore data for this Ligand-Target Pair
TargetIntegrase(Human immunodeficiency virus 1)
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Inhibition of HIV1 integraseMore data for this Ligand-Target Pair
TargetIntegrase(Human immunodeficiency virus 1)
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: 0.600nMAssay Description:Inhibition of pseudotype HIV1 integrase strand transfer activity assessed as reduction in viral replication in HIV1 infected human CIP4 cells after 2...More data for this Ligand-Target Pair
TargetIntegrase(Human immunodeficiency virus 1)
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: 0.700nMAssay Description:Inhibition of pseudotype HIV1 integrase strand transfer activity assessed as reduction in viral replication in HIV1 infected human CIP4 cells after 2...More data for this Ligand-Target Pair
TargetIntegrase(Human immunodeficiency virus 1)
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of pseudotype HIV1 integrase strand transfer activity assessed as reduction in viral replication in in HIV1 infected human CIP4 cells afte...More data for this Ligand-Target Pair
TargetIntegrase(Human immunodeficiency virus 1)
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of HIV1 integrase strand transfer by biochemical assayMore data for this Ligand-Target Pair
TargetIntegrase(Human immunodeficiency virus 1)
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: 1.80nMAssay Description:Inhibition of HIV1 integraseMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:In vitro concentration required to inhibit the overall HIV-1 integrase strand transferMore data for this Ligand-Target Pair
TargetIntegrase(Human immunodeficiency virus 1)
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of HIV1 integrase strand transfer activityMore data for this Ligand-Target Pair
TargetIntegrase(Human immunodeficiency virus 1)
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of pseudotype HIV1 integrase strand transfer activity assessed as reduction in viral replication in in HIV1 infected human CIP4 cells afte...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l...More data for this Ligand-Target Pair
TargetIntegrase(Human immunodeficiency virus 1)
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of pseudotype HIV1 integrase strand transfer activity assessed as reduction in viral replication in HIV1 infected human CIP4 cells after 2...More data for this Ligand-Target Pair
TargetIntegrase(Human immunodeficiency virus 1)
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of HIV integrase strand transfer activityMore data for this Ligand-Target Pair
TargetIntegrase(Human immunodeficiency virus 1)
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of HIV1 integrase by overall integration assayMore data for this Ligand-Target Pair

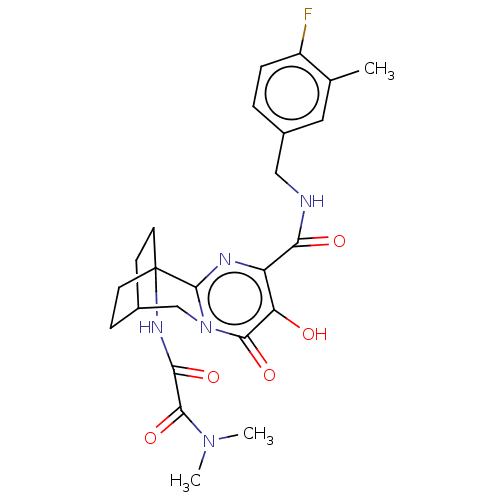
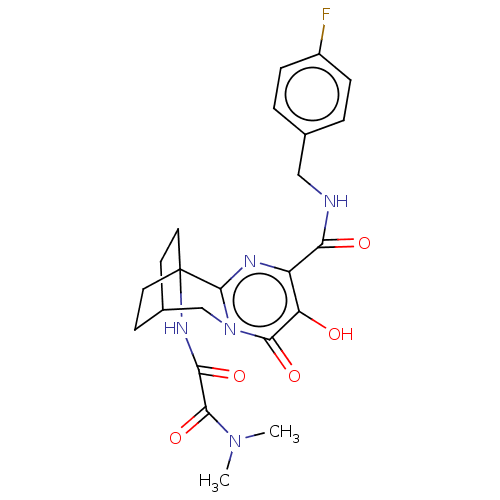
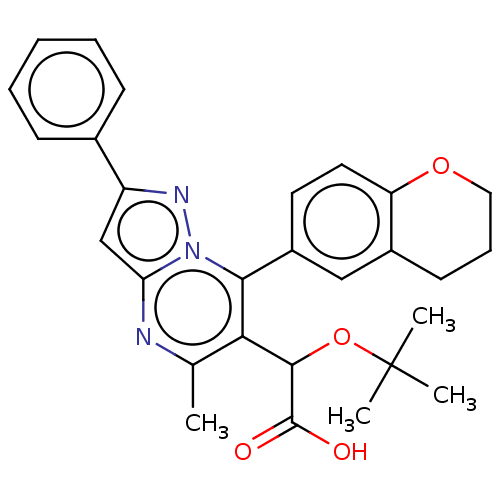


 3D Structure (crystal)
3D Structure (crystal)