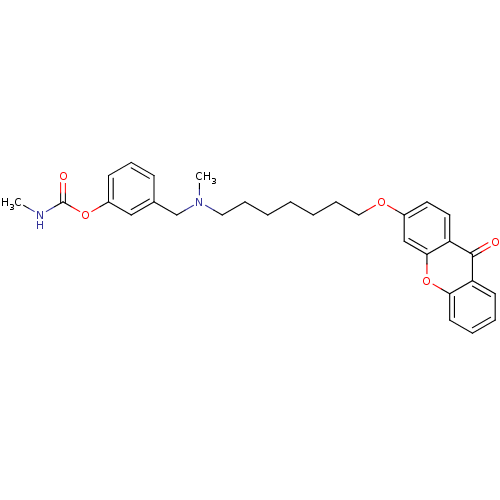
Affinity DataIC50: 0.320nMAssay Description:Inhibition of human AChEMore data for this Ligand-Target Pair
Affinity DataIC50: 0.320nMpH: 8.0 T: 2°CAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.320nMpH: 8.0 T: 2°CAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.320nMAssay Description:Inhibition of human serum BChE using butyrylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.320nMAssay Description:Inhibition of human erythrocyte AchEMore data for this Ligand-Target Pair
Affinity DataIC50: 16.5nMAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 16.5nMAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 16.5nMAssay Description:Inhibition of human serum BuChEMore data for this Ligand-Target Pair
Affinity DataIC50: 52nMAssay Description:Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 30 mins followed by substrate addition measured aft...More data for this Ligand-Target Pair