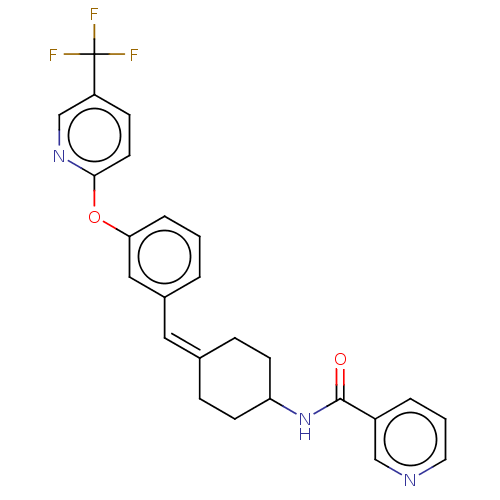
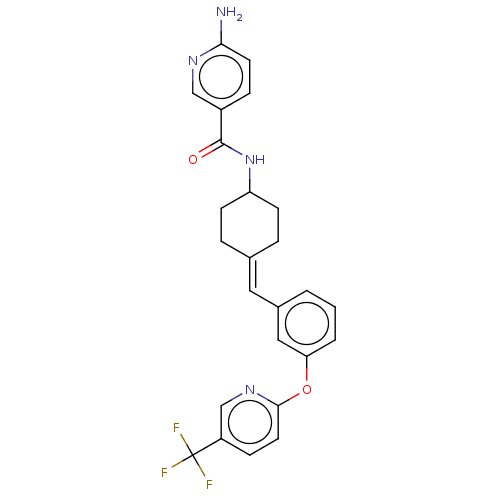
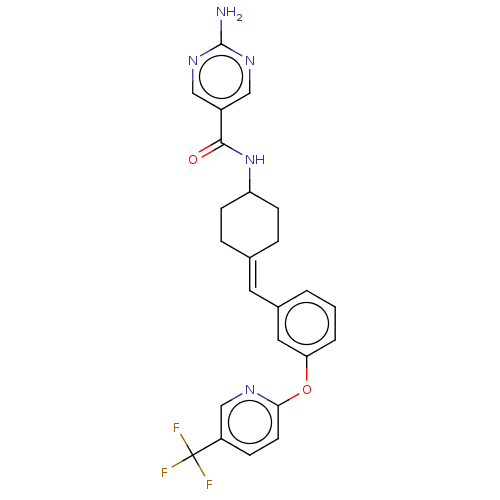
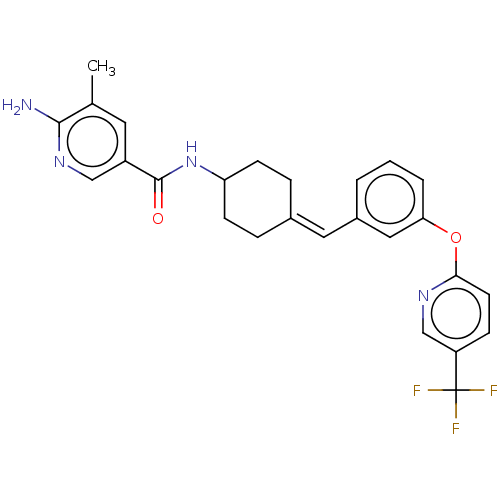
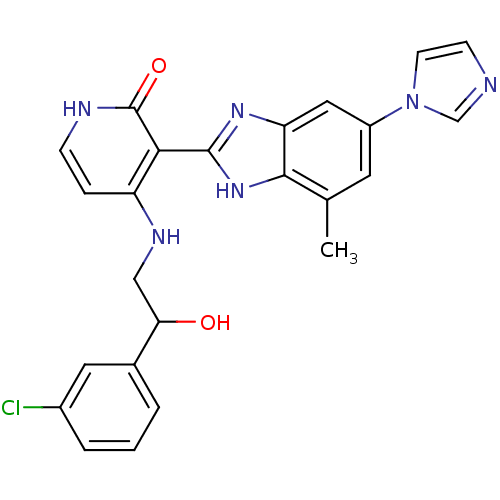
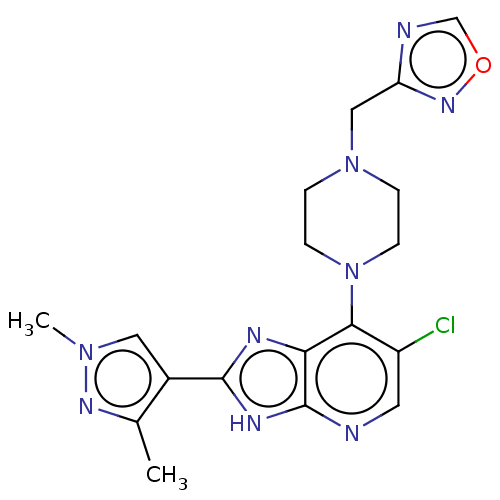
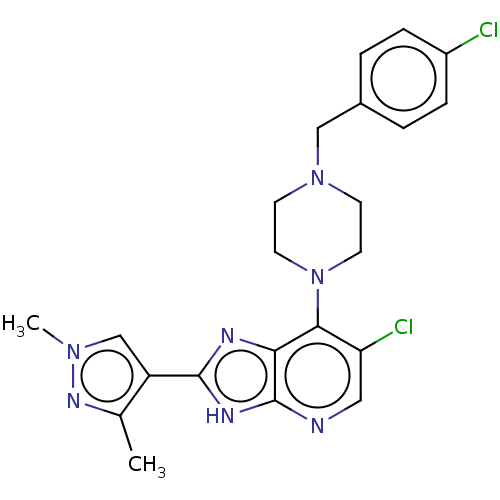
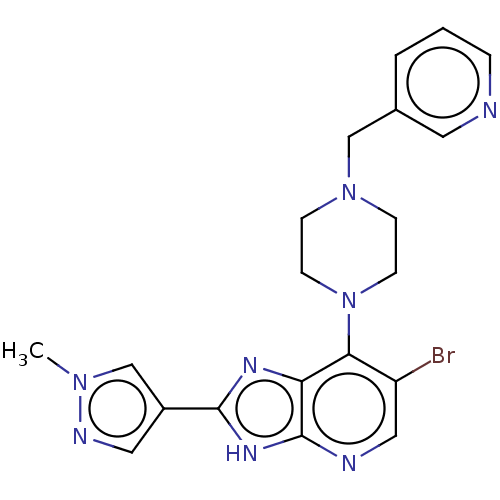
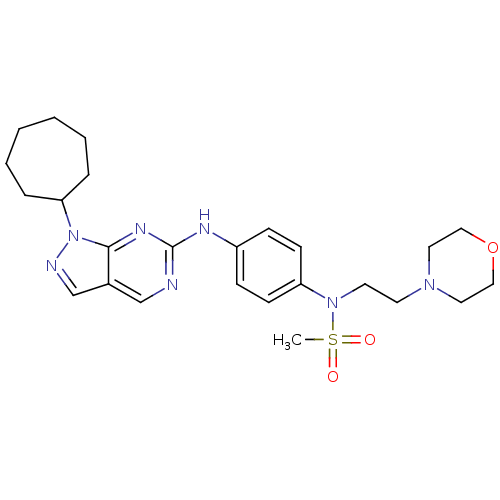
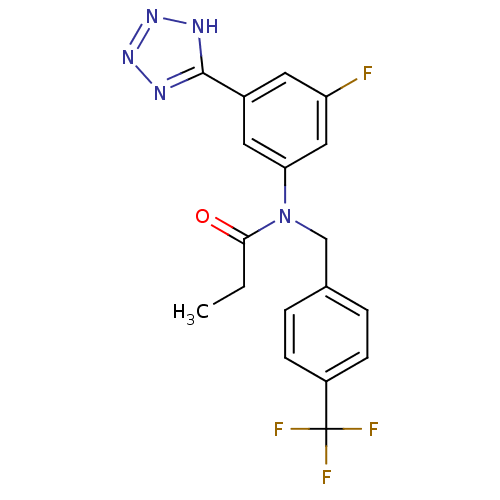
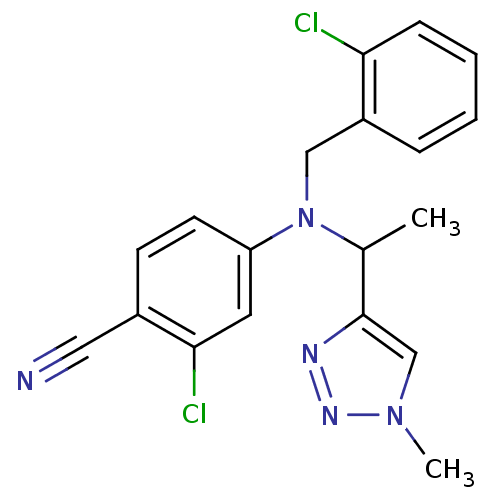
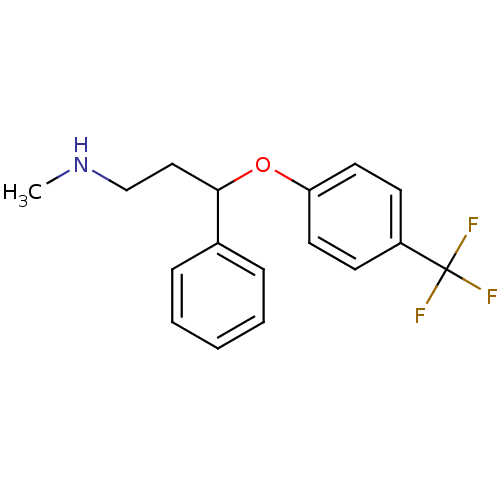
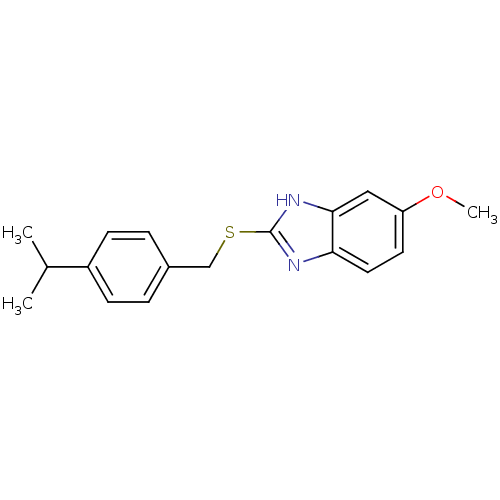
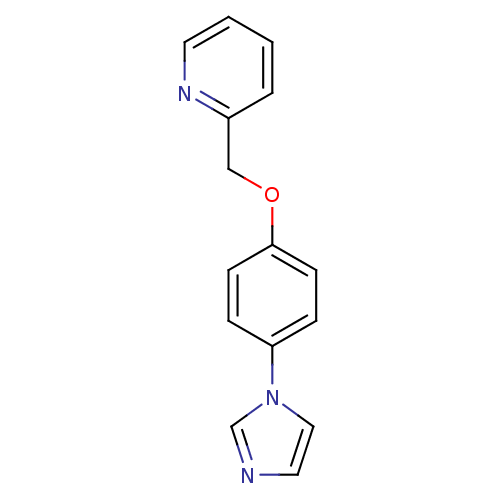
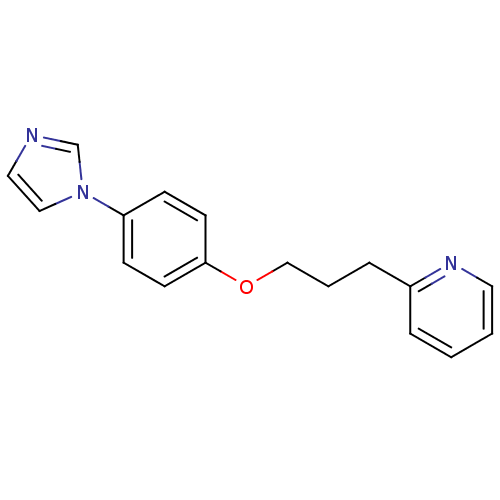
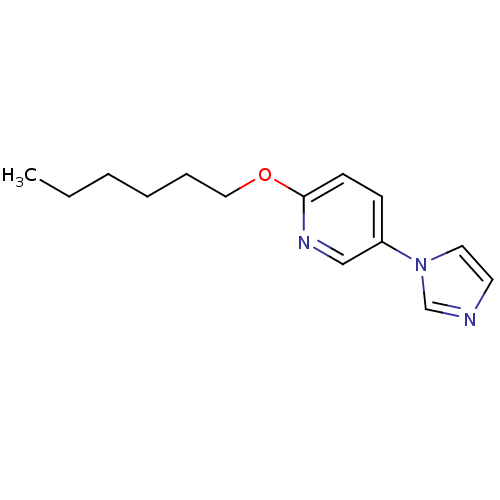
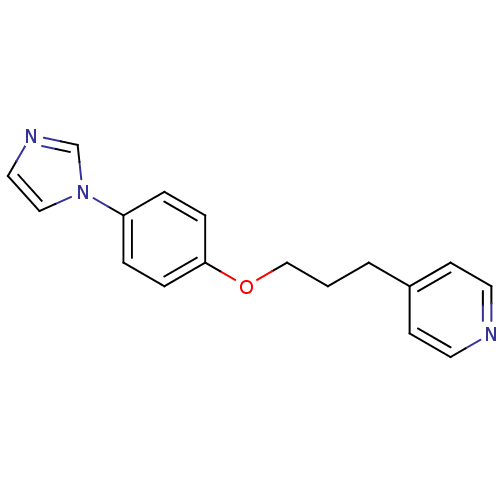
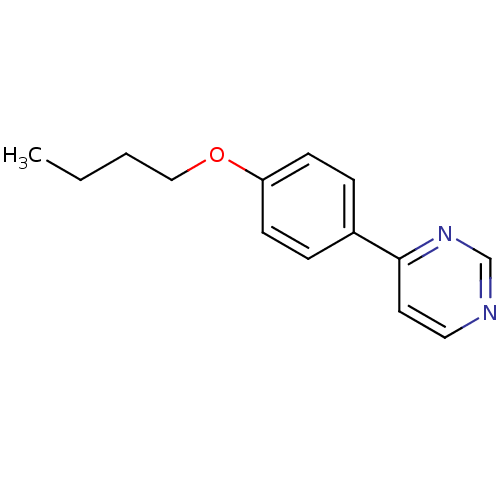
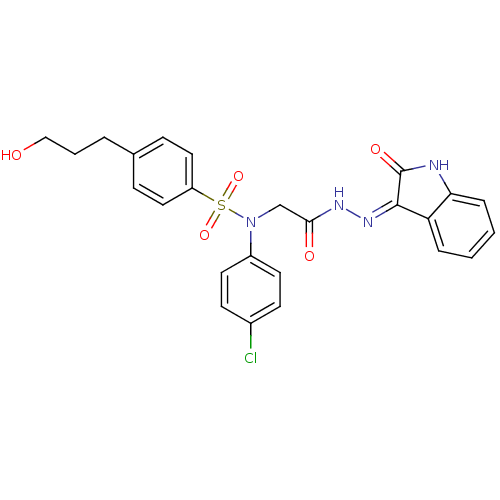
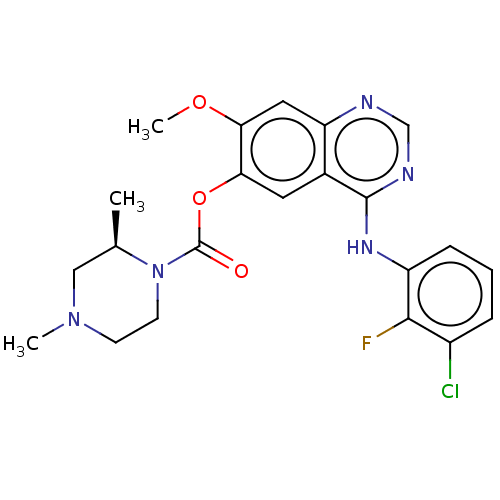
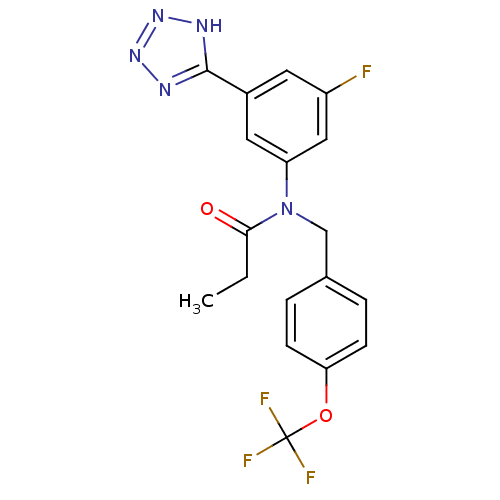
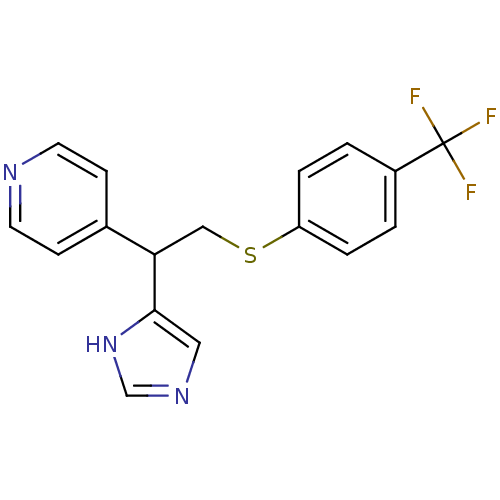
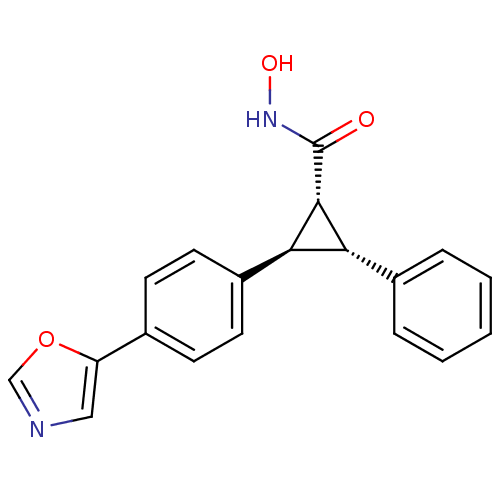
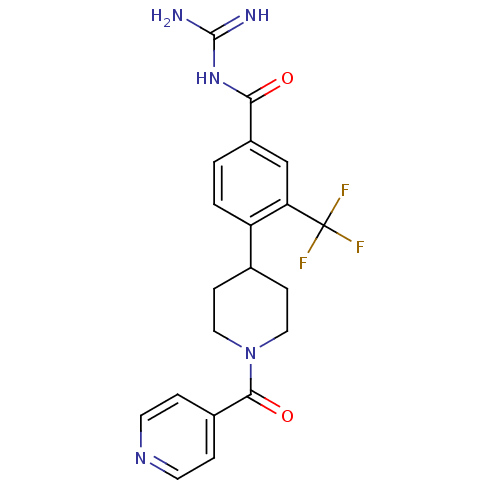
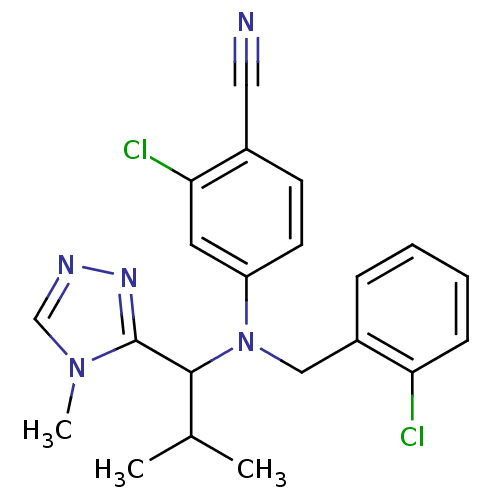
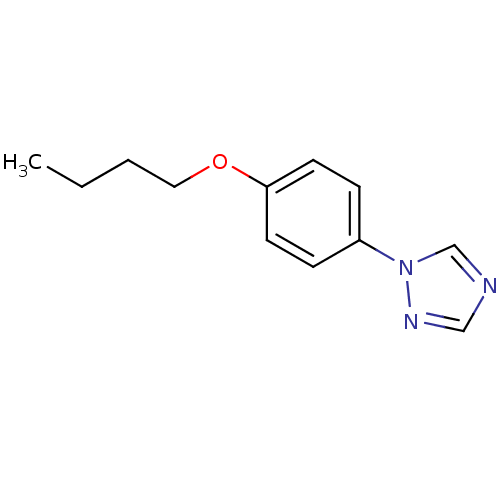
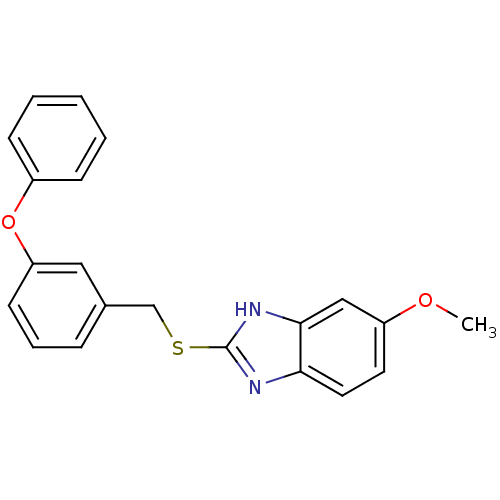
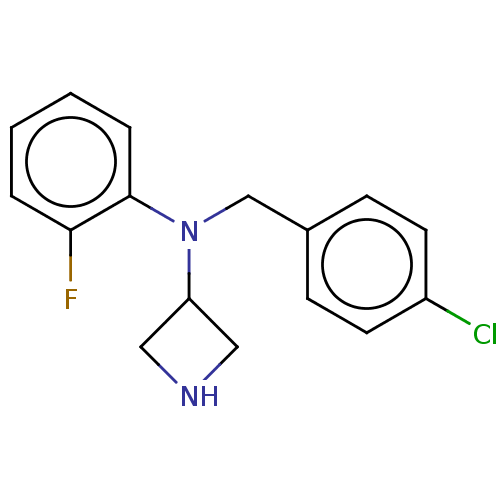
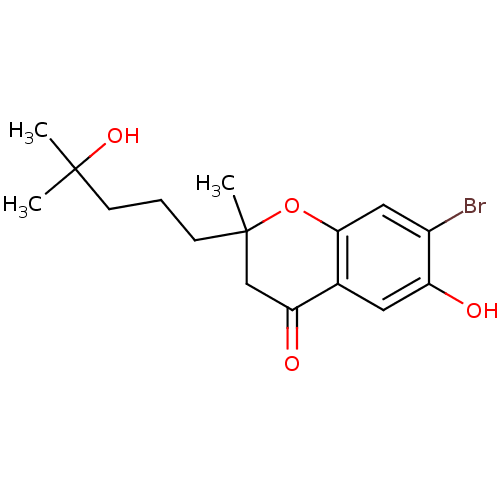
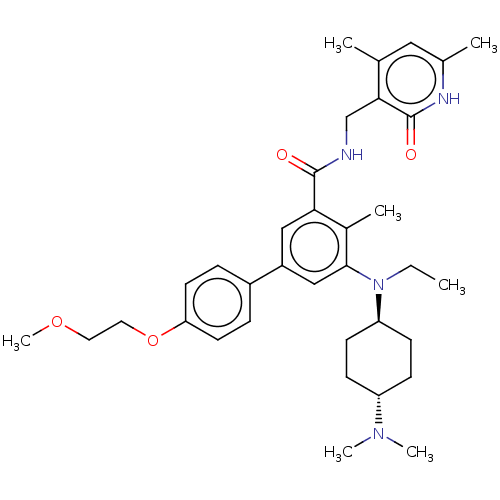
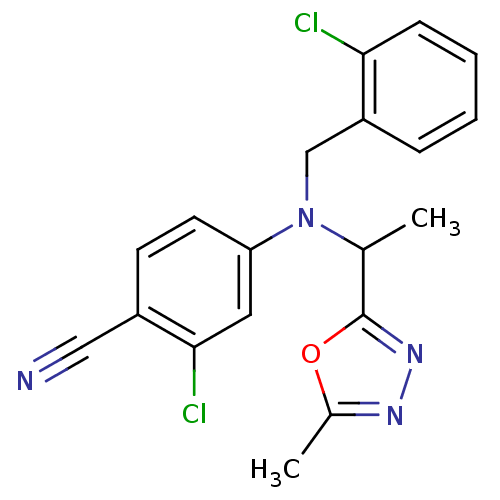
Affinity DataIC50: 4.60nMAssay Description:Inhibition of CYP2C19 in human liver microsomes using (S)-mephenytoin as substrate preincubated for 5 mins followed by NADPH cofactor addition and me...More data for this Ligand-Target Pair
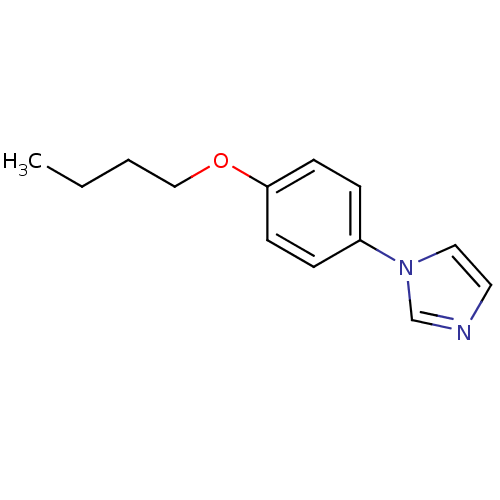
Affinity DataIC50: 7nMAssay Description:Concentration required to inhibit cytochrome P450 19A1.More data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Inhibition of cytochrome P450 2C19More data for this Ligand-Target Pair
Affinity DataIC50: 8.5nMAssay Description:Inhibition of CYP2C19More data for this Ligand-Target Pair
Affinity DataIC50: 8.5nMAssay Description:Inhibition of human CYP2C19 expressed in insect cell microsome after 30 mins by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 9.10nMAssay Description:Inhibition of CYP2C19 in human liver microsomes using (S)-mephenytoin as substrate preincubated for 5 mins followed by NADPH cofactor addition and me...More data for this Ligand-Target Pair
Affinity DataIC50: >13nMAssay Description:Inhibition of CYP2C19 in human liver microsomes using (S)-mephenytoin as substrate preincubated for 5 mins followed by NADPH cofactor addition and me...More data for this Ligand-Target Pair
Affinity DataIC50: >13nMAssay Description:Inhibition of CYP2C19 in human liver microsomes using (S)-mephenytoin as substrate preincubated for 5 mins followed by NADPH cofactor addition and me...More data for this Ligand-Target Pair
Affinity DataIC50: >20nMAssay Description:Inhibition of CYP2C19 in human liver microsomesMore data for this Ligand-Target Pair
Affinity DataIC50: 26nMAssay Description:Concentration required to inhibit cytochrome P450 isozyme CYP2C19 in vitro by 50%More data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibition of recombinant CYP2C19 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibition of recombinant CYP2C19 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 33.6nMAssay Description:The potential inhibition of enzyme activities of human cytochromes P450 (CYP) of Compound 1, 2, or 105 was evaluated using pooled human liver microso...More data for this Ligand-Target Pair
Affinity DataIC50: 38nMAssay Description:Inhibition of human recombinant CYP2C19 incubated for 15 mins prior to substrate addition measured after 30 mins by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:Inhibition of CYP2C19 (unknown origin) using luciferin tagged substrate preincubated for 10 mins before substrate additionMore data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:Inhibition of CYP2C19 in human liver microsomes using (S)-mpheytoin as substrate preincubated for 5 mins by LC-MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:Inhibition of CYP2C19 in human liver microsomes incubated for 5 mins in presence of NADPH and specific substrates by LC/MS/MS methodMore data for this Ligand-Target Pair
Affinity DataIC50: <46nMAssay Description:Inhibition of cytochrome P450 2C19More data for this Ligand-Target Pair
Affinity DataIC50: <46nMAssay Description:Inhibition of cytochrome P450 2C19More data for this Ligand-Target Pair
Affinity DataIC50: <46nMAssay Description:Inhibition of cytochrome P450 2C19More data for this Ligand-Target Pair
Affinity DataIC50: <46nMAssay Description:Inhibition of cytochrome P450 2C19More data for this Ligand-Target Pair
Affinity DataIC50: >46nMAssay Description:Concentration required to inhibit cytochrome P450 19A1.More data for this Ligand-Target Pair
Affinity DataIC50: <46nMAssay Description:Inhibition of cytochrome P450 2C19More data for this Ligand-Target Pair
Affinity DataIC50: >46nMAssay Description:Concentration required to inhibit cytochrome P450 19A1.More data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:Inhibitory activity against cytochrome P450 2C19 isoformMore data for this Ligand-Target Pair
Affinity DataIC50: >50nMAssay Description:Inhibition of CYP2C19 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >50nMAssay Description:Inhibition of CYP2C19More data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:Inhibition of CYP2C19 in human liver microsomes using (S)-mpheytoin as substrate preincubated for 5 mins by LC-MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 51nMAssay Description:Inhibition of human recombinant CYP2C19 incubated for 15 mins prior to substrate addition measured after 30 mins by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 60nMAssay Description:Inhibition of human CYP2C19More data for this Ligand-Target Pair
Affinity DataIC50: 60nMAssay Description:Inhibiton of CYP2C19 in human liver microsomes using 7-ethoxy-3-cyanocoumarin as substrate after 45 mins by LC/MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 65nMAssay Description:Inhibition of CYP2C19 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 68nMAssay Description:Concentration required to inhibit cytochrome P450 19A1.More data for this Ligand-Target Pair
Affinity DataIC50: 70nMAssay Description:Inhibition of CYP2C19 in human liver microsomes using (S)-mpheytoin as substrate preincubated for 5 mins by LC-MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 70nMAssay Description:Inhibition of human recombinant CYP2C19 incubated for 5 mins by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 80nMAssay Description:The test compounds were evaluated for their ability to inhibit the catlytic activity of human CYP1 enzymes by means of high throughput fluorometric d...More data for this Ligand-Target Pair
Affinity DataIC50: 89.6nMAssay Description:The potential inhibition of enzyme activities of human cytochromes P450 (CYP) of Compound 1, 2, or 105 was evaluated using pooled human liver microso...More data for this Ligand-Target Pair
Affinity DataIC50: 91nMAssay Description:Inhibition of cytochrome P450 2C19More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Inhibition of human CYP2C19More data for this Ligand-Target Pair