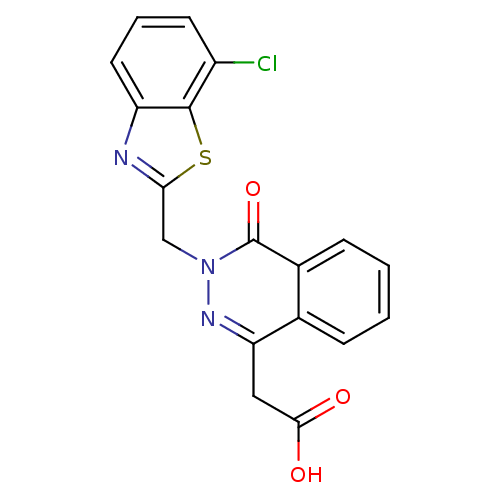
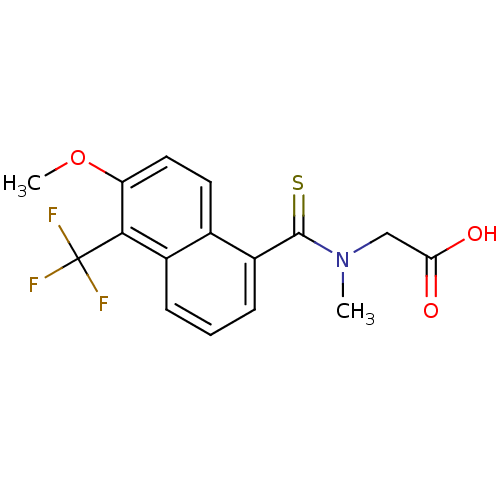
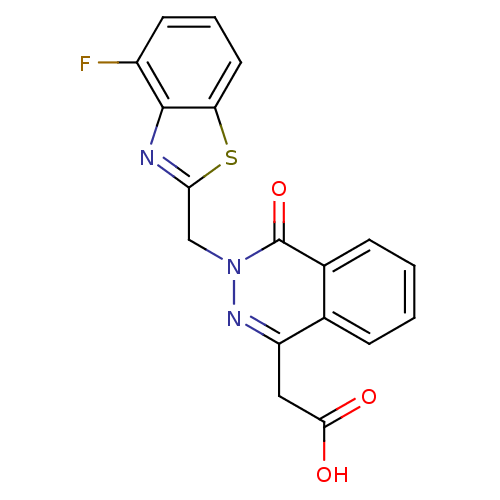
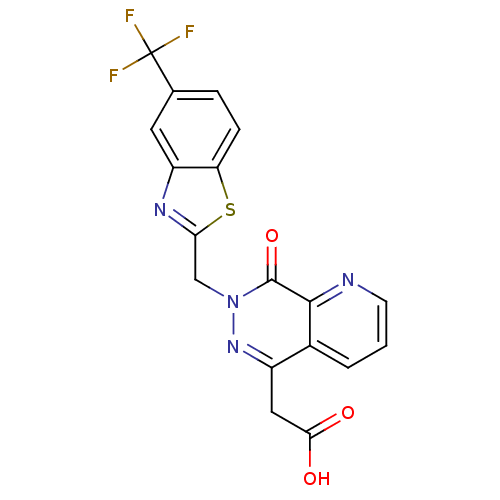
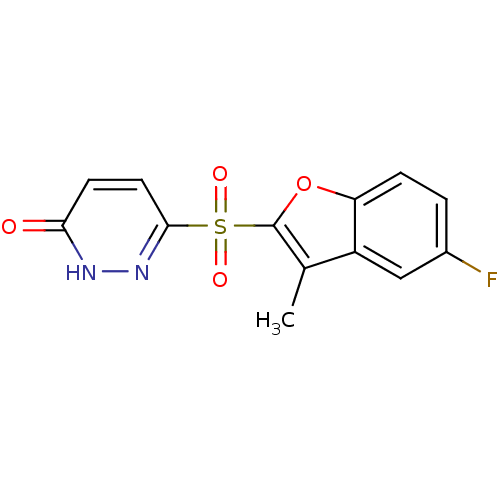
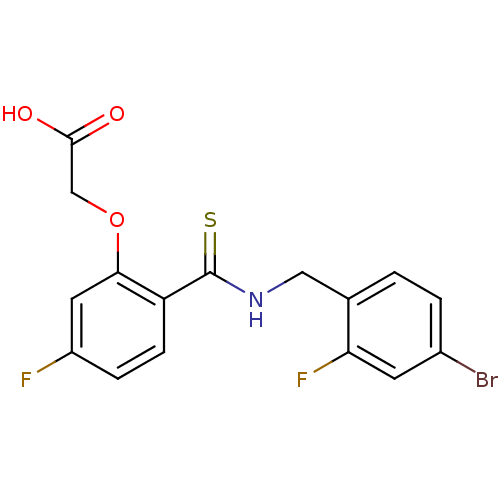
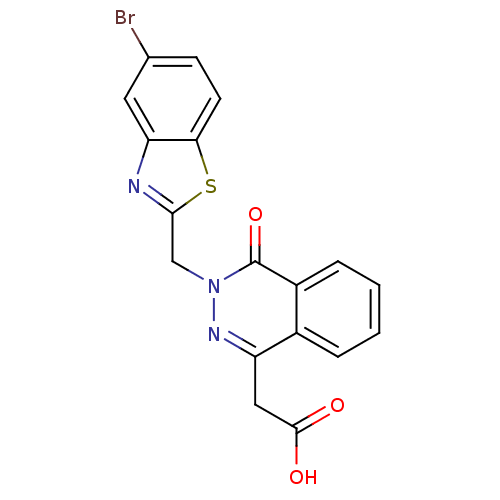
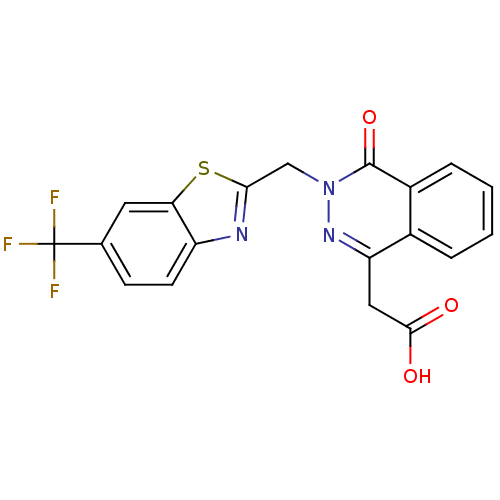
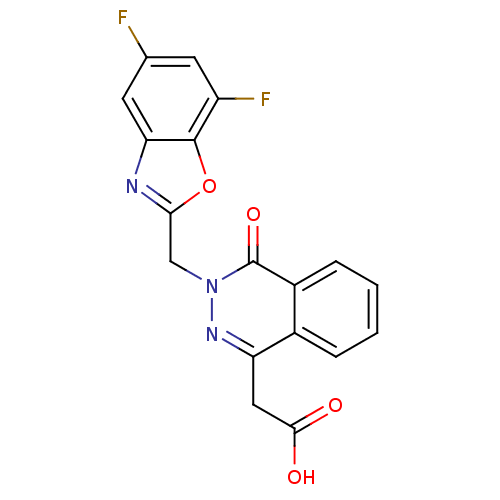
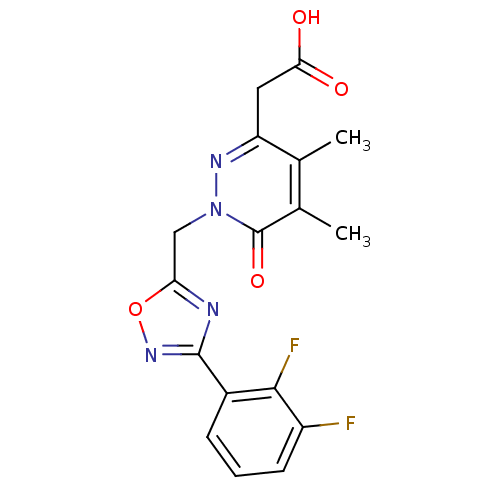
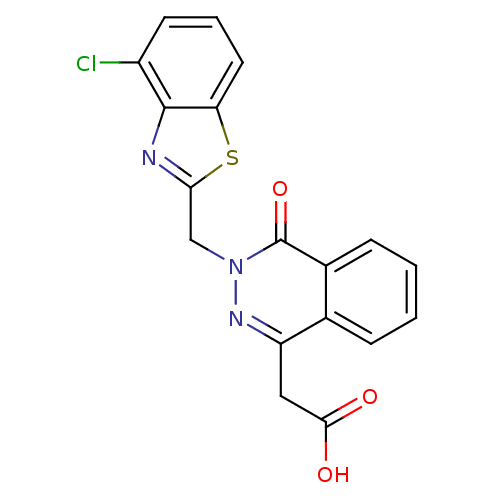
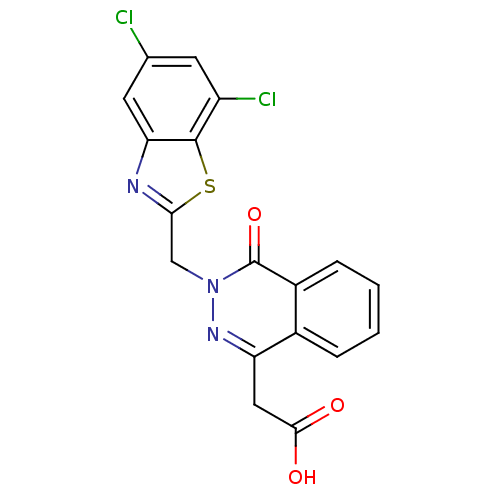
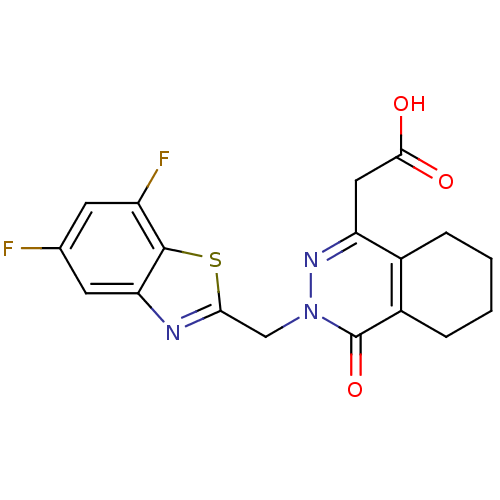
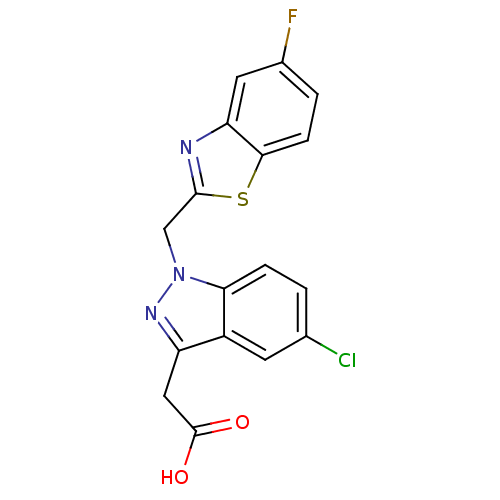
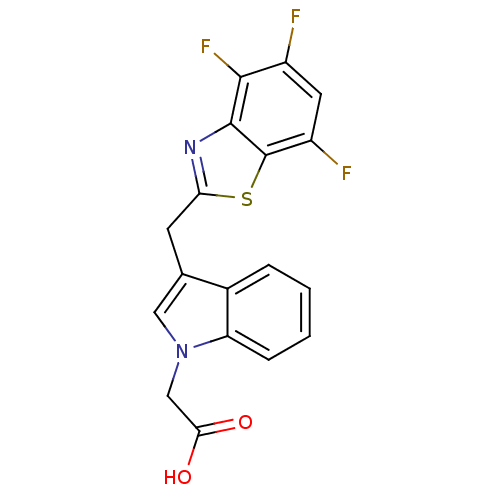
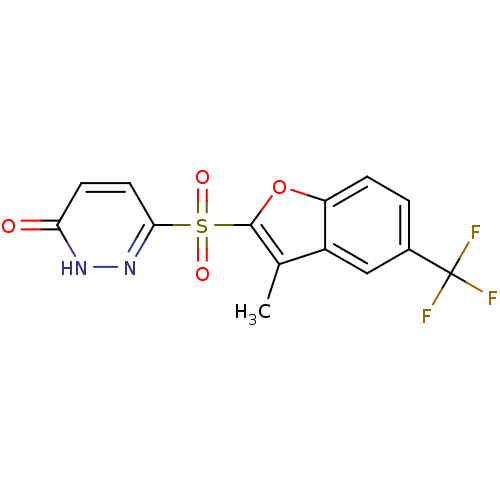
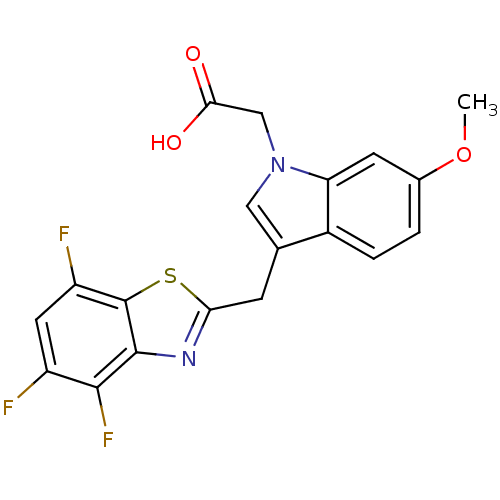
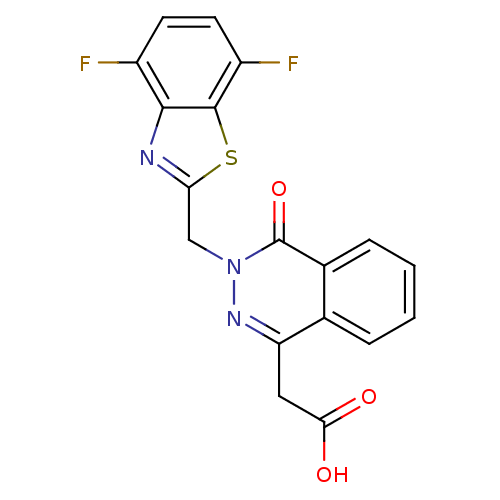
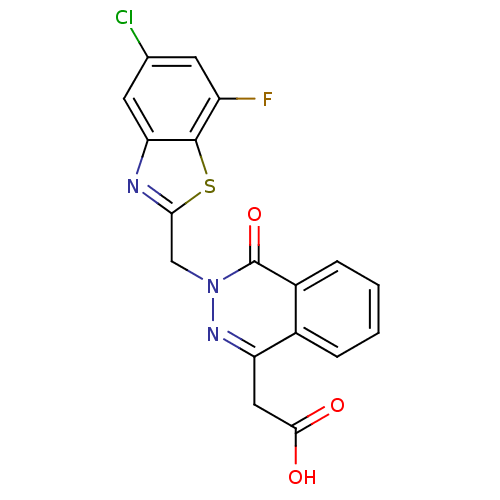
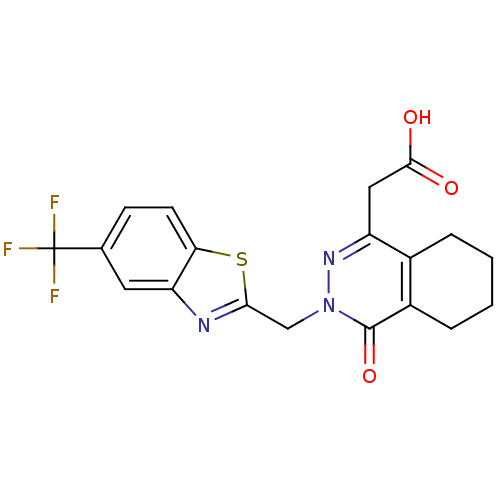
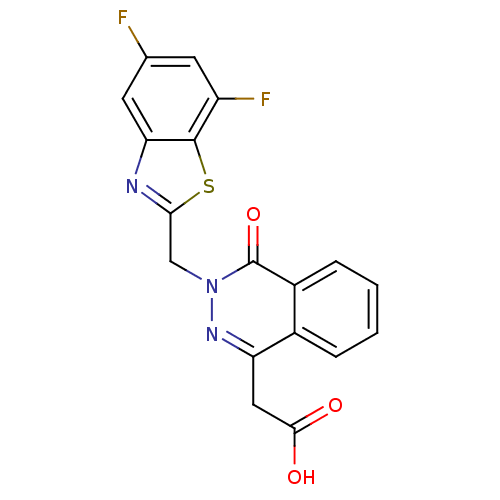
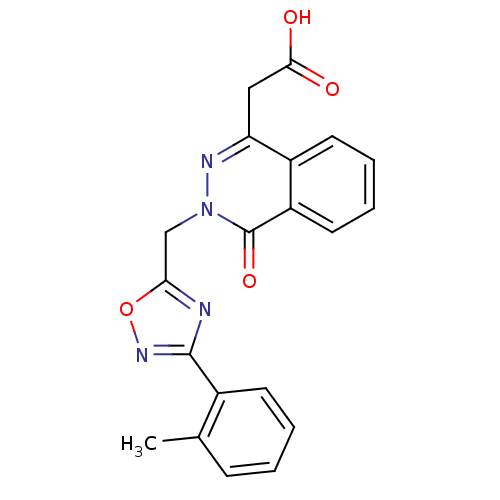
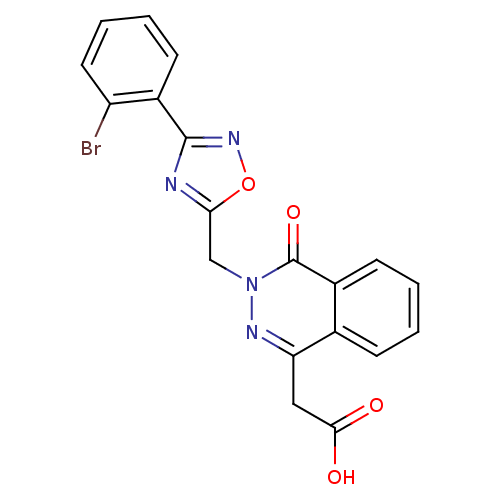
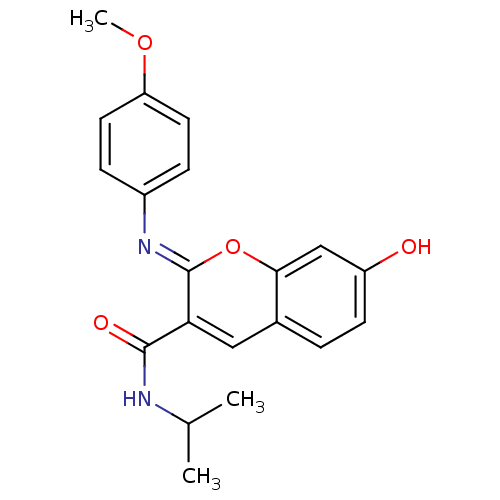
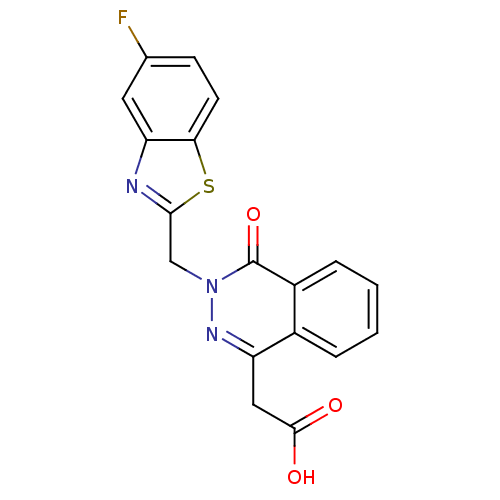
Affinity DataIC50: 0.840nMAssay Description:Inhibition of Homo sapiens (human) recombinant aldose reductase using D-glyceraldehyde as substrate incubated for 10 min prior to substrate addition ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.840nMpH: 7.0 T: 2°CAssay Description:The activity of the enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappearance ...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:The activity of the enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappearance ...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of aldose reductase (aldo-keto reductase, AKR1B1) isolated from human placenta.More data for this Ligand-Target Pair
Affinity DataIC50: 1nMpH: 7.0Assay Description:The IC50-activity assays were carried out on the basis of the quantification of the NADPH consumption that takes place when the enzyme catalyzes the ...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of aldose reductaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.90nMAssay Description:Compound was tested for the rate of reduction of glyceraldehyde by human placental aldose reductase.More data for this Ligand-Target Pair
Affinity DataIC50: 1.90nMAssay Description:Compound was tested for the inhibition of the human placental aldose reductase using the substrate as glyceraldehyde.More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of aldose reductase (aldo-keto reductase, AKR1B1) isolated from human placenta.More data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:Inhibitory activity against aldose reductase isolated from human placentaMore data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:Inhibition of human placenta Aldose reductaseMore data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:Inhibition of human Aldose reductaseMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:The activity of the enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappearance ...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMpH: 7.0Assay Description:The IC50-activity assays were carried out on the basis of the quantification of the NADPH consumption that takes place when the enzyme catalyzes the ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.10nMAssay Description:Compound was tested for the inhibition of the rat lens aldose reductase using the substrate as glucose.More data for this Ligand-Target Pair
Affinity DataIC50: 3.10nMAssay Description:Inhibition of human placental aldose reductase using glyceraldehyde as substrate in presence of NADPHMore data for this Ligand-Target Pair
Affinity DataIC50: 3.10nMAssay Description:In vitro inhibitory activity against aldose reductase isolated from human placentaMore data for this Ligand-Target Pair
Affinity DataIC50: 3.10nMAssay Description:Inhibitory activity against aldose reductase enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 3.10nMAssay Description:Inhibition of human placenta Aldose reductaseMore data for this Ligand-Target Pair
Affinity DataIC50: 3.10nMAssay Description:Inhibition of aldose reductase (aldo-keto reductase, AKR1B1) isolated from human placenta.More data for this Ligand-Target Pair
Affinity DataIC50: 3.10nMAssay Description:Inhibition of aldose reductaseMore data for this Ligand-Target Pair
Affinity DataIC50: 3.20nMAssay Description:In vitro inhibitory activity against aldose reductase isolated from human placentaMore data for this Ligand-Target Pair
Affinity DataIC50: 3.20nMAssay Description:Inhibition of human placenta Aldose reductaseMore data for this Ligand-Target Pair
Affinity DataIC50: 3.20nMAssay Description:Inhibition of human Aldose reductaseMore data for this Ligand-Target Pair
Affinity DataIC50: 3.30nMAssay Description:Inhibitory activity against aldose reductase isolated from human placentaMore data for this Ligand-Target Pair
Affinity DataIC50: 3.5nMAssay Description:Inhibition of aldose reductase (aldo-keto reductase, AKR1B1) isolated from human placenta.More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of aldose reductase (aldo-keto reductase, AKR1B1) isolated from human placenta.More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibitory activity against aldose reductase isolated from human placentaMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMpH: 7.0 T: 2°CAssay Description:The activity of the enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappearance ...More data for this Ligand-Target Pair
Affinity DataIC50: 4.60nMAssay Description:Inhibitory activity against aldose reductase isolated from human placentaMore data for this Ligand-Target Pair
Affinity DataIC50: 4.80nMAssay Description:Inhibitory activity against aldose reductase isolated from human placentaMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibitory activity against aldose reductase isolated from human placentaMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMpH: 6.6 T: 2°CAssay Description:The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:The activity of the enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappearance ...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of aldose reductaseMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of aldose reductase (aldo-keto reductase, AKR1B1) isolated from human placenta.More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of aldose reductase (aldo-keto reductase, AKR1B1) isolated from human placenta.More data for this Ligand-Target Pair
Affinity DataIC50: 5.20nMAssay Description:Inhibitory activity against aldose reductase isolated from human placentaMore data for this Ligand-Target Pair
Affinity DataIC50: 5.20nMAssay Description:Inhibition of human Aldose reductaseMore data for this Ligand-Target Pair
Affinity DataIC50: 5.90nMAssay Description:Inhibition of aldose reductase (aldo-keto reductase, AKR1B1) isolated from human placenta.More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:The activity of the enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappearance ...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of aldose reductaseMore data for this Ligand-Target Pair
Affinity DataIC50: 6.20nMAssay Description:Inhibition of aldose reductase (aldo-keto reductase, AKR1B1) isolated from human placenta.More data for this Ligand-Target Pair
Affinity DataIC50: 6.30nMAssay Description:Inhibition of aldose reductase (aldo-keto reductase, AKR1B1) isolated from human placenta.More data for this Ligand-Target Pair
Affinity DataIC50: 6.40nMAssay Description:In vitro inhibitory activity against aldose reductase isolated from human placentaMore data for this Ligand-Target Pair
Affinity DataIC50: 6.5nMAssay Description:In vitro inhibitory activity against aldose reductase isolated from human placentaMore data for this Ligand-Target Pair
Affinity DataIC50: 6.70nMAssay Description:Inhibition of human recombinant N-terminal His6-tagged AKR1B1 expressed in Escherichia coli by fluorescence assay in presence of NADPHMore data for this Ligand-Target Pair
Affinity DataIC50: 6.90nMAssay Description:Inhibition of aldose reductase (aldo-keto reductase, AKR1B1) isolated from human placenta.More data for this Ligand-Target Pair

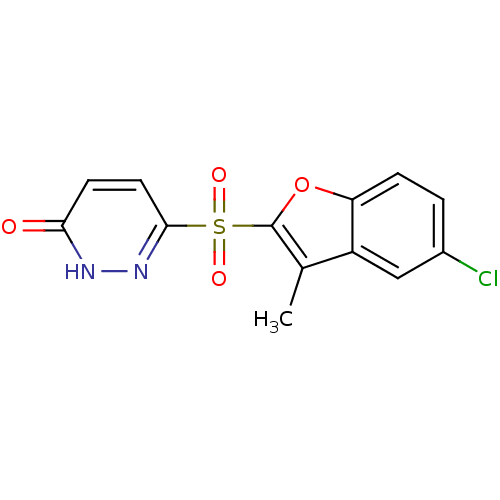
 3D Structure (crystal)
3D Structure (crystal)