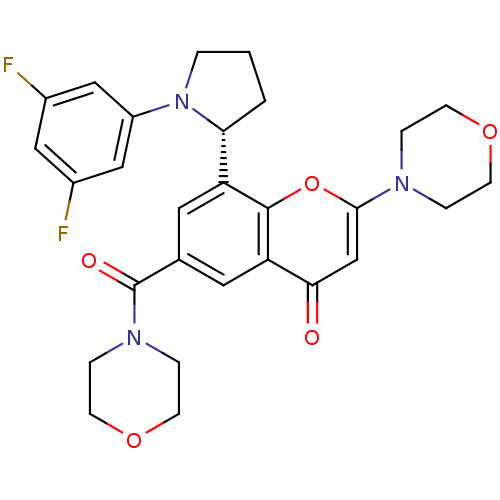
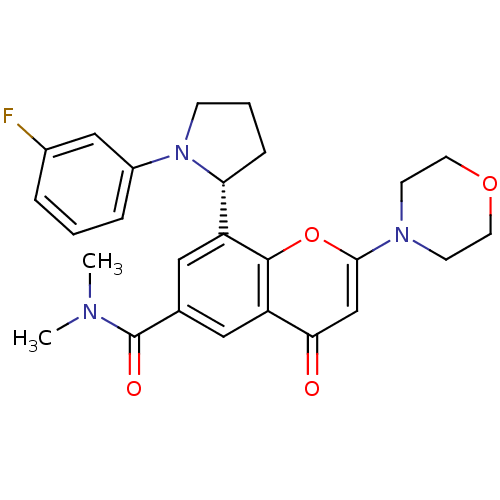
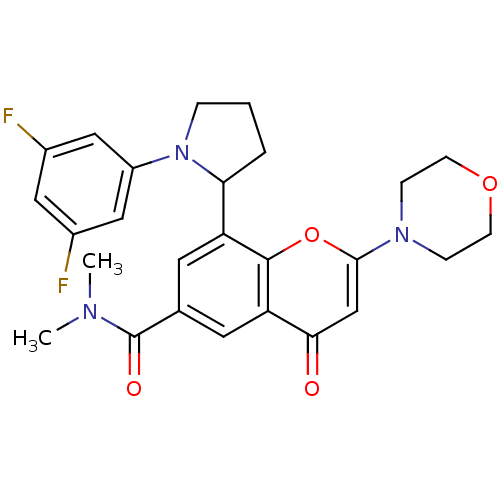
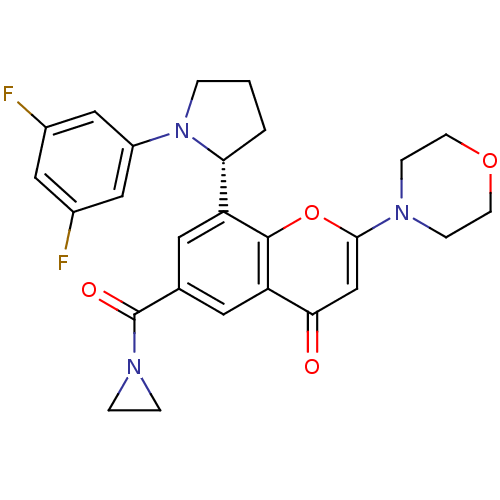
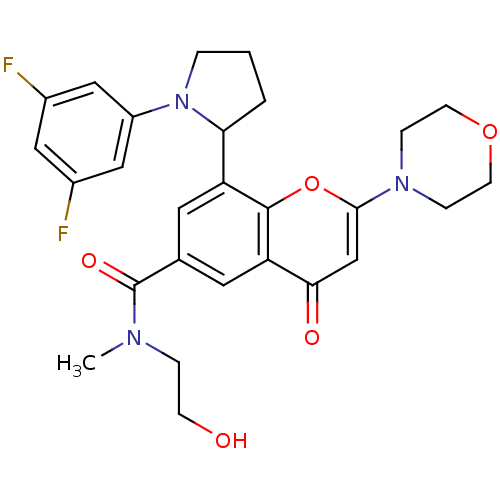
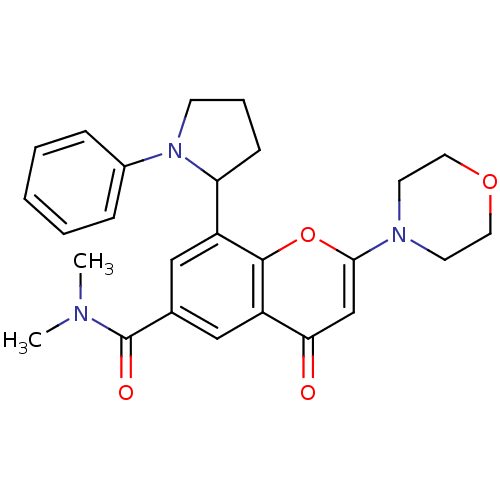
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Astrazeneca
Curated by ChEMBL
Astrazeneca
Curated by ChEMBL
Affinity DataIC50: 1.10nMAssay Description:Inhibition of PI3Kbeta in PTEN-deficient human MDA-MB-468 cells assessed as decrease in AKT phosphorylation at Ser473 measured after 2 hrsMore data for this Ligand-Target Pair
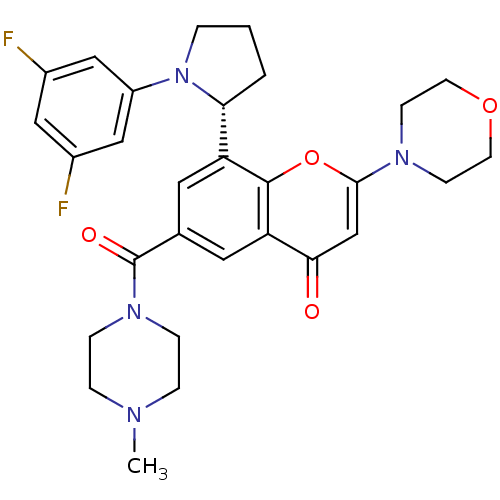
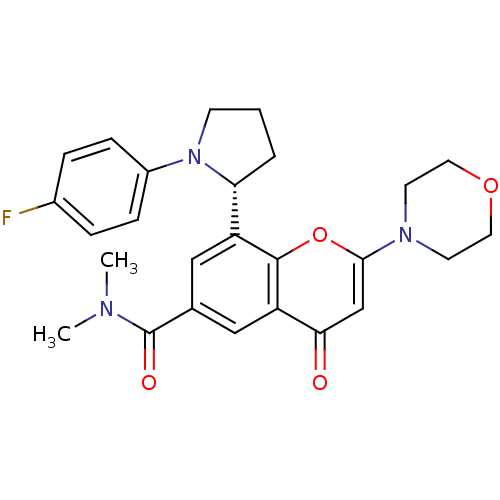
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Astrazeneca
Curated by ChEMBL
Astrazeneca
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:Inhibition of PI3Kbeta in PTEN-deficient human MDA-MB-468 cells assessed as decrease in AKT phosphorylation at Ser473 measured after 2 hrsMore data for this Ligand-Target Pair
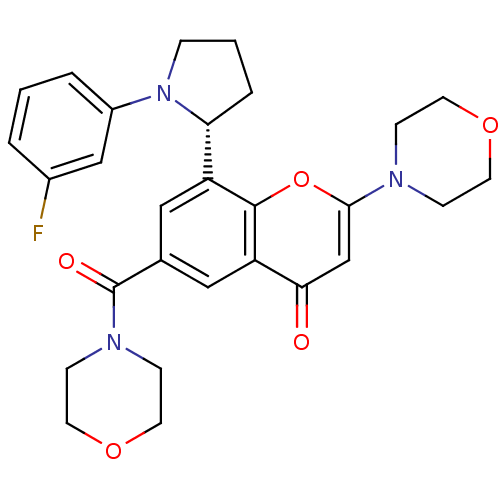
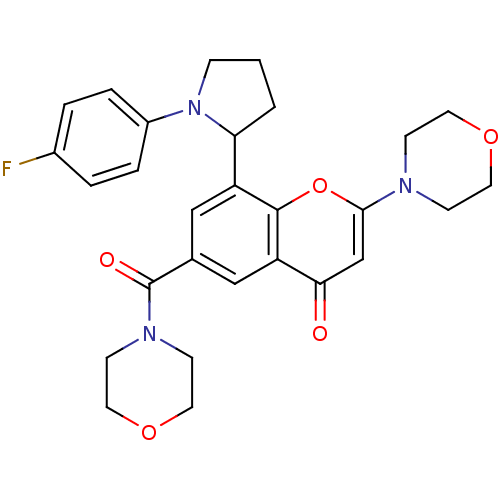
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Astrazeneca
Curated by ChEMBL
Astrazeneca
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:Inhibition of PI3Kbeta in PTEN-deficient human MDA-MB-468 cells assessed as decrease in AKT phosphorylation at Ser473 measured after 2 hrsMore data for this Ligand-Target Pair
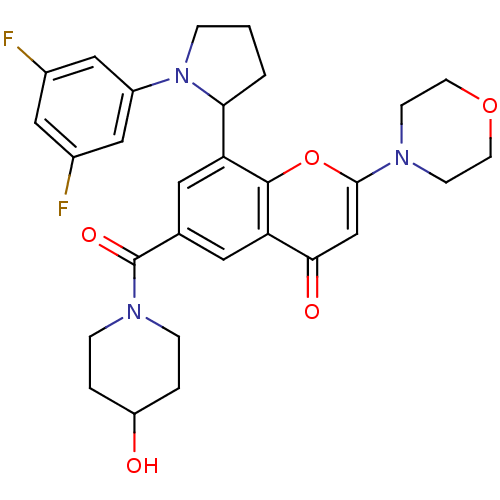
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Astrazeneca
Curated by ChEMBL
Astrazeneca
Curated by ChEMBL
Affinity DataIC50: 1.40nMAssay Description:Inhibition of PI3Kbeta in PTEN-deficient human MDA-MB-468 cells assessed as decrease in AKT phosphorylation at Ser473 measured after 2 hrsMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Astrazeneca
Curated by ChEMBL
Astrazeneca
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of PI3Kbeta in PTEN-deficient human MDA-MB-468 cells assessed as decrease in AKT phosphorylation at Ser473 measured after 2 hrsMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Astrazeneca
Curated by ChEMBL
Astrazeneca
Curated by ChEMBL
Affinity DataIC50: 2.30nMAssay Description:Inhibition of PI3Kbeta in PTEN-deficient human MDA-MB-468 cells assessed as decrease in AKT phosphorylation at Ser473 measured after 2 hrsMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Astrazeneca
Curated by ChEMBL
Astrazeneca
Curated by ChEMBL
Affinity DataIC50: 2.80nMAssay Description:Inhibition of PI3Kbeta in PTEN-deficient human MDA-MB-468 cells assessed as decrease in AKT phosphorylation at Ser473 measured after 2 hrsMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Astrazeneca
Curated by ChEMBL
Astrazeneca
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of PI3Kbeta in PTEN-deficient human MDA-MB-468 cells assessed as decrease in AKT phosphorylation at Ser473 measured after 2 hrsMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Astrazeneca
Curated by ChEMBL
Astrazeneca
Curated by ChEMBL
Affinity DataIC50: 3.20nMAssay Description:Inhibition of PI3Kbeta in PTEN-deficient human MDA-MB-468 cells assessed as decrease in AKT phosphorylation at Ser473 measured after 2 hrsMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Astrazeneca
Curated by ChEMBL
Astrazeneca
Curated by ChEMBL
Affinity DataIC50: 3.40nMAssay Description:Inhibition of PI3Kbeta in PTEN-deficient human MDA-MB-468 cells assessed as decrease in AKT phosphorylation at Ser473 measured after 2 hrsMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Astrazeneca
Curated by ChEMBL
Astrazeneca
Curated by ChEMBL
Affinity DataIC50: 4.5nMAssay Description:Inhibition of PI3Kbeta in PTEN-deficient human MDA-MB-468 cells assessed as decrease in AKT phosphorylation at Ser473 measured after 2 hrsMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Astrazeneca
Curated by ChEMBL
Astrazeneca
Curated by ChEMBL
Affinity DataIC50: 5.10nMAssay Description:Displacement of [3H]baclofen from Gamma-aminobutyric acid type B receptor of rat brain membranesMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Astrazeneca
Curated by ChEMBL
Astrazeneca
Curated by ChEMBL
Affinity DataIC50: 6.10nMAssay Description:Inhibition of recombinant human PI3Kbeta using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 80 mins in pr...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Astrazeneca
Curated by ChEMBL
Astrazeneca
Curated by ChEMBL
Affinity DataIC50: 6.70nMAssay Description:Inhibition of recombinant human PI3Kbeta using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 80 mins in pr...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Astrazeneca
Curated by ChEMBL
Astrazeneca
Curated by ChEMBL
Affinity DataIC50: 7.10nMAssay Description:Inhibition of recombinant human PI3Kbeta using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 80 mins in pr...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Astrazeneca
Curated by ChEMBL
Astrazeneca
Curated by ChEMBL
Affinity DataIC50: 8.20nMAssay Description:Inhibition of recombinant human PI3Kdelta using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 80 mins in p...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Astrazeneca
Curated by ChEMBL
Astrazeneca
Curated by ChEMBL
Affinity DataIC50: 8.60nMAssay Description:Inhibition of recombinant human PI3Kdelta using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 80 mins in p...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Astrazeneca
Curated by ChEMBL
Astrazeneca
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Inhibition of recombinant human PI3Kalpha using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 80 mins in p...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Astrazeneca
Curated by ChEMBL
Astrazeneca
Curated by ChEMBL
Affinity DataIC50: 14nMAssay Description:Inhibition of PI3Kdelta in human JeKo1 B cells assessed as decrease in AKT phosphorylation at Ser473 measured after 1 hrMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Astrazeneca
Curated by ChEMBL
Astrazeneca
Curated by ChEMBL
Affinity DataIC50: 19nMAssay Description:Inhibition of recombinant human PI3Kalpha using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 80 mins in p...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Astrazeneca
Curated by ChEMBL
Astrazeneca
Curated by ChEMBL
Affinity DataIC50: 24nMAssay Description:Inhibition of recombinant human PI3Kalpha using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 80 mins in p...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Astrazeneca
Curated by ChEMBL
Astrazeneca
Curated by ChEMBL
Affinity DataIC50: 190nMAssay Description:Compound was evaluated for Kinetic constant for viral thymidine kinase of Herpes simplex virus (HSV) -2More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Astrazeneca
Curated by ChEMBL
Astrazeneca
Curated by ChEMBL
Affinity DataIC50: 330nMAssay Description:Inhibition of recombinant human PI3Kgamma using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 80 mins in p...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Astrazeneca
Curated by ChEMBL
Astrazeneca
Curated by ChEMBL
Affinity DataIC50: 340nMAssay Description:Inhibition of recombinant human PI3Kgamma using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 80 mins in p...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Mus musculus (Mouse))
Astrazeneca
Curated by ChEMBL
Astrazeneca
Curated by ChEMBL
Affinity DataIC50: 1.50E+3nMAssay Description:Displacement of [3H]baclofen from Gamma-aminobutyric acid type B receptor of rat brain membranesMore data for this Ligand-Target Pair
Affinity DataIC50: 2.90E+4nMAssay Description:Inhibition of human SphK1 (1 to 384 residues) by ATP-Glo HTS assayMore data for this Ligand-Target Pair
Affinity DataIC50: >3.00E+4nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >3.00E+4nMAssay Description:Displacement of [3H]baclofen from Gamma-aminobutyric acid type B receptor of rat brain membranesMore data for this Ligand-Target Pair
Affinity DataIC50: >3.00E+4nMAssay Description:Displacement of [3H]baclofen from Gamma-aminobutyric acid type B receptor of rat brain membranesMore data for this Ligand-Target Pair
Affinity DataIC50: >3.00E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >3.00E+4nMAssay Description:Inhibition of CYP2C19 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 4.20E+4nMAssay Description:Inhibition of mTOR (unknown origin)More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Astrazeneca
Curated by ChEMBL
Astrazeneca
Curated by ChEMBL
Affinity DataIC50: 4.50E+4nMAssay Description:Displacement of [3H]baclofen from Gamma-aminobutyric acid type B receptor of rat brain membranesMore data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of human CHKalpha (1 to 457 residues) by ADP Glo HTS assayMore data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Displacement of [3H]baclofen from Gamma-aminobutyric acid type B receptor of rat brain membranesMore data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Displacement of [3H]baclofen from Gamma-aminobutyric acid type B receptor of rat brain membranesMore data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Displacement of [3H]baclofen from Gamma-aminobutyric acid type B receptor of rat brain membranesMore data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Displacement of [3H]baclofen from Gamma-aminobutyric acid type B receptor of rat brain membranesMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha(Homo sapiens (Human))
Astrazeneca
Curated by ChEMBL
Astrazeneca
Curated by ChEMBL
Affinity DataIC50: >5.00E+4nMAssay Description:Displacement of [3H]baclofen from Gamma-aminobutyric acid type B receptor of rat brain membranesMore data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of human PIK4Cbeta (1 to 801 residues) by ADP Glo HTS assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit beta(Homo sapiens (Human))
Astrazeneca
Curated by ChEMBL
Astrazeneca
Curated by ChEMBL
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of human PI3K p85beta (1 to 724 residues) by ADP Glo HTS assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Astrazeneca
Curated by ChEMBL
Astrazeneca
Curated by ChEMBL
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of human PI3Kgamma (2 to 1102 residues) by ADP Glo HTS assayMore data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of human SPHK 2 (1 to 654 residues) by ADP Glo HTS assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 5-phosphate 4-kinase type-2 alpha(Homo sapiens)
Astrazeneca
Curated by ChEMBL
Astrazeneca
Curated by ChEMBL
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of human PIP5K2alpha (1 to 406 residues) by ADP Glo HTS assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 5-phosphate 4-kinase type-2 alpha(Homo sapiens)
Astrazeneca
Curated by ChEMBL
Astrazeneca
Curated by ChEMBL
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of human PI4K2alpha (1 to 479 residues) by ADP Glo HTS assayMore data for this Ligand-Target Pair