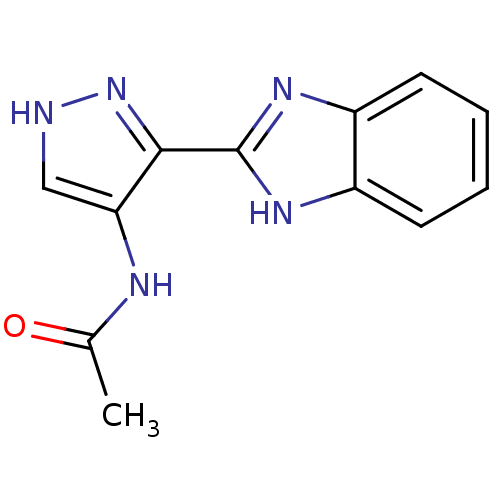
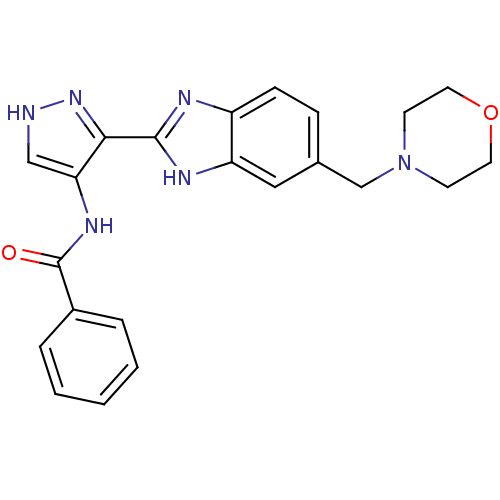
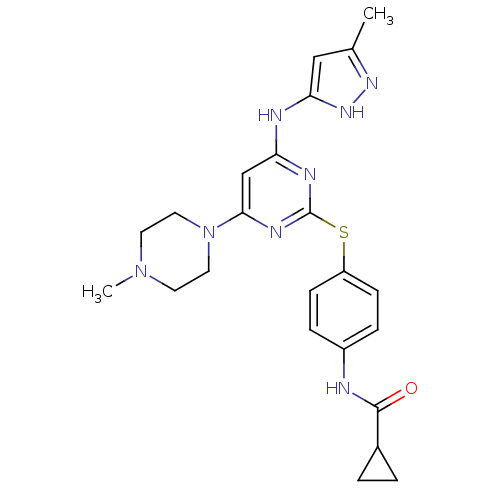
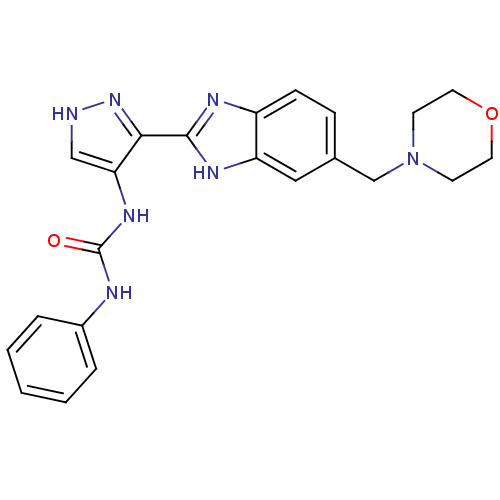
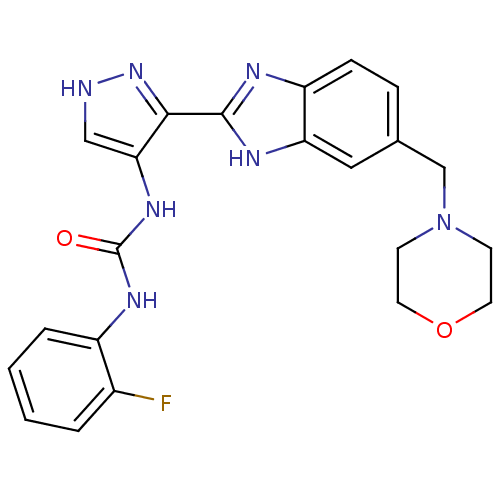
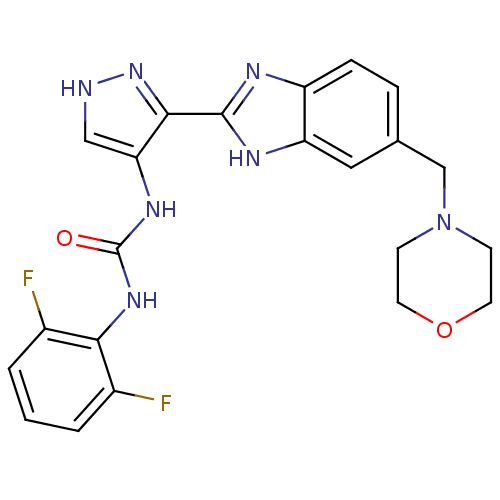
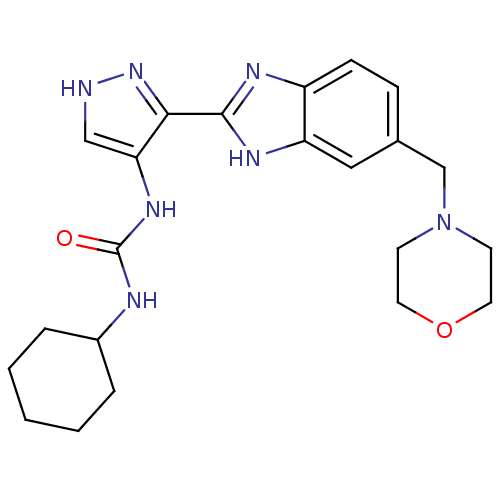
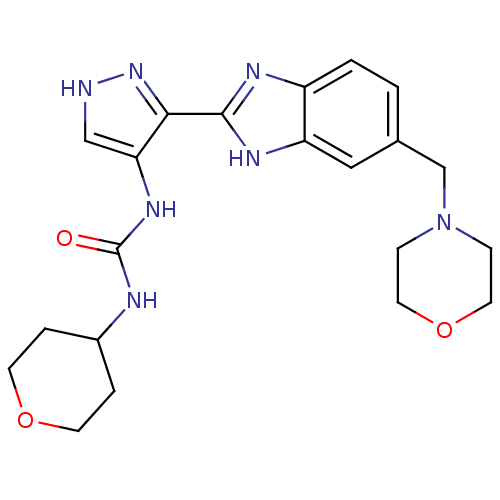
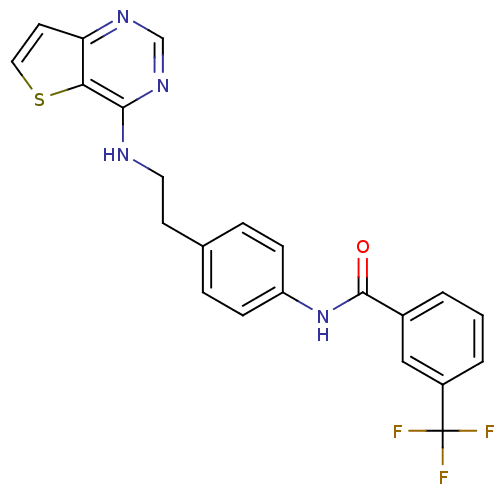
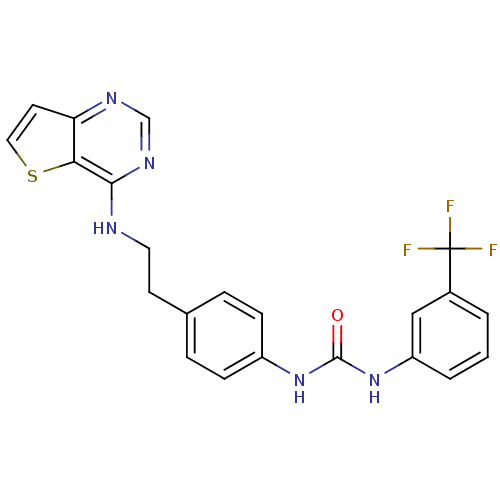
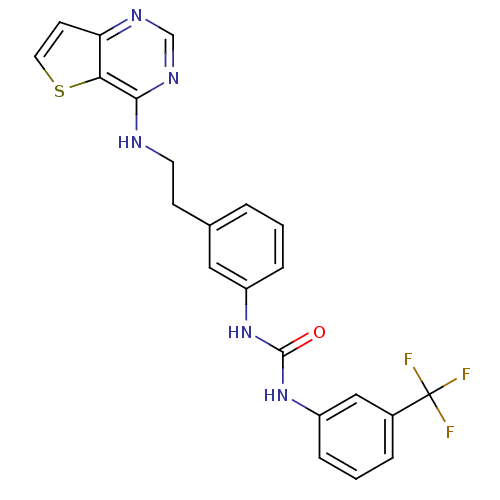
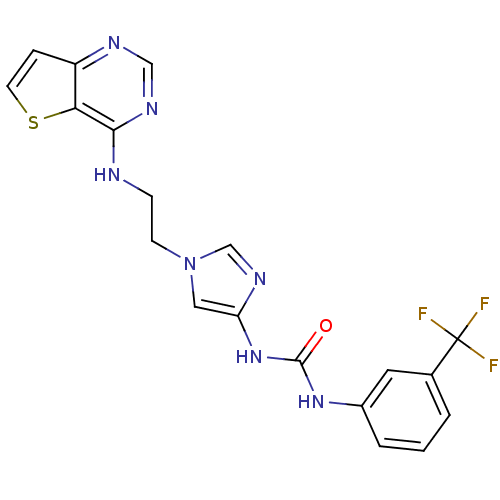
Affinity DataIC50: >1.00E+4nMpH: 7.0 T: 2°CAssay Description:The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMpH: 7.0 T: 2°CAssay Description:Aurora-A (h) is incubated with 8 mM MOPS pH 7.0, 0.2 mM EDTA, 200 μM LRRASLG (Kemptide), 10 mM MgAcetate and [γ-33P-ATP](specific activity ...More data for this Ligand-Target Pair
Affinity DataIC50: 400nMpH: 7.0 T: 2°CAssay Description:The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMpH: 7.0 T: 2°CAssay Description:The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio...More data for this Ligand-Target Pair
Affinity DataIC50: 500nMpH: 7.0 T: 2°CAssay Description:The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMpH: 7.0 T: 2°CAssay Description:The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+3nMpH: 7.0 T: 2°CAssay Description:The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio...More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+3nMpH: 7.0 T: 2°CAssay Description:The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio...More data for this Ligand-Target Pair
Affinity DataIC50: 2.90E+3nMpH: 7.0 T: 2°CAssay Description:The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMpH: 7.0 T: 2°CAssay Description:The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio...More data for this Ligand-Target Pair
Affinity DataIC50: 2.84E+3nMpH: 7.0 T: 2°CAssay Description:The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio...More data for this Ligand-Target Pair
Affinity DataIC50: 2.40E+3nMpH: 7.0 T: 2°CAssay Description:The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio...More data for this Ligand-Target Pair
Affinity DataIC50: 2.10E+3nMpH: 7.0 T: 2°CAssay Description:The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio...More data for this Ligand-Target Pair
Affinity DataIC50: 510nMpH: 7.0 T: 2°CAssay Description:The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio...More data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+3nMpH: 7.0 T: 2°CAssay Description:The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio...More data for this Ligand-Target Pair
Affinity DataIC50: 1.72E+4nMpH: 7.0 T: 2°CAssay Description:The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio...More data for this Ligand-Target Pair
Affinity DataIC50: 1.40E+3nMpH: 7.0 T: 2°CAssay Description:The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio...More data for this Ligand-Target Pair
Affinity DataIC50: 900nMpH: 7.0 T: 2°CAssay Description:The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio...More data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+3nMpH: 7.0 T: 2°CAssay Description:The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+4nMpH: 7.0 T: 2°CAssay Description:The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio...More data for this Ligand-Target Pair
Affinity DataIC50: 600nMpH: 7.0 T: 2°CAssay Description:The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio...More data for this Ligand-Target Pair
Affinity DataIC50: 9.20E+3nMpH: 7.0 T: 2°CAssay Description:The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio...More data for this Ligand-Target Pair
Affinity DataIC50: 360nMpH: 7.0 T: 2°CAssay Description:The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio...More data for this Ligand-Target Pair
Affinity DataIC50: 5.70E+3nMpH: 7.0 T: 2°CAssay Description:The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio...More data for this Ligand-Target Pair
Affinity DataIC50: 79nMpH: 7.0 T: 2°CAssay Description:The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio...More data for this Ligand-Target Pair
Affinity DataIC50: 140nMpH: 7.0 T: 2°CAssay Description:The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio...More data for this Ligand-Target Pair
Affinity DataIC50: 1.48E+3nMpH: 7.0 T: 2°CAssay Description:The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio...More data for this Ligand-Target Pair
Affinity DataIC50: 97nMpH: 7.0 T: 2°CAssay Description:The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio...More data for this Ligand-Target Pair
Affinity DataIC50: 330nMpH: 7.0 T: 2°CAssay Description:The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio...More data for this Ligand-Target Pair
Affinity DataIC50: 980nMpH: 7.0 T: 2°CAssay Description:The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio...More data for this Ligand-Target Pair
Affinity DataIC50: 910nMpH: 7.0 T: 2°CAssay Description:Assays for Aurora kinase was performed in a DELFIA format. Aurora enzyme was incubated with test compound and cross-tide substrate in the reaction bu...More data for this Ligand-Target Pair
Affinity DataIC50: 70nMpH: 7.0 T: 2°CAssay Description:Assays for Aurora kinase was performed in a DELFIA format. Aurora enzyme was incubated with test compound and cross-tide substrate in the reaction bu...More data for this Ligand-Target Pair
Affinity DataIC50: 5.90nMpH: 7.0 T: 2°CAssay Description:Assays for Aurora kinase was performed in a DELFIA format. Aurora enzyme was incubated with test compound and cross-tide substrate in the reaction bu...More data for this Ligand-Target Pair
Affinity DataIC50: 3.5nMpH: 7.0 T: 2°CAssay Description:Assays for Aurora kinase was performed in a DELFIA format. Aurora enzyme was incubated with test compound and cross-tide substrate in the reaction bu...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMpH: 7.0 T: 2°CAssay Description:Assays for Aurora kinase was performed in a DELFIA format. Aurora enzyme was incubated with test compound and cross-tide substrate in the reaction bu...More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMpH: 7.0 T: 2°CAssay Description:Assays for Aurora kinase was performed in a DELFIA format. Aurora enzyme was incubated with test compound and cross-tide substrate in the reaction bu...More data for this Ligand-Target Pair
Affinity DataIC50: 12nMpH: 7.0 T: 2°CAssay Description:Assays for Aurora kinase was performed in a DELFIA format. Aurora enzyme was incubated with test compound and cross-tide substrate in the reaction bu...More data for this Ligand-Target Pair
Affinity DataIC50: 2.80nMpH: 7.0 T: 2°CAssay Description:Assays for Aurora kinase was performed in a DELFIA format. Aurora enzyme was incubated with test compound and cross-tide substrate in the reaction bu...More data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMpH: 7.0 T: 2°CAssay Description:Assays for Aurora kinase was performed in a DELFIA format. Aurora enzyme was incubated with test compound and cross-tide substrate in the reaction bu...More data for this Ligand-Target Pair
Affinity DataIC50: 5.70nMpH: 7.0 T: 2°CAssay Description:Assays for Aurora kinase was performed in a DELFIA format. Aurora enzyme was incubated with test compound and cross-tide substrate in the reaction bu...More data for this Ligand-Target Pair
Affinity DataIC50: 13nMpH: 7.0 T: 2°CAssay Description:Assays for Aurora kinase was performed in a DELFIA format. Aurora enzyme was incubated with test compound and cross-tide substrate in the reaction bu...More data for this Ligand-Target Pair
Affinity DataIC50: 26nMpH: 7.0 T: 2°CAssay Description:Assays for Aurora kinase was performed in a DELFIA format. Aurora enzyme was incubated with test compound and cross-tide substrate in the reaction bu...More data for this Ligand-Target Pair
Affinity DatapH: 7.0 T: 2°CAssay Description:Assays for Aurora kinase was performed in a DELFIA format. Aurora enzyme was incubated with test compound and cross-tide substrate in the reaction bu...More data for this Ligand-Target Pair
Affinity DataIC50: 22nMpH: 7.0 T: 2°CAssay Description:Aurora-A (h) is incubated with 8 mM MOPS pH 7.0, 0.2 mM EDTA, 200 μM LRRASLG (Kemptide), 10 mM MgAcetate and [γ-33P-ATP](specific activity ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+3nMpH: 7.0 T: 2°CAssay Description:The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio...More data for this Ligand-Target Pair
Affinity DataIC50: 3.20E+3nMpH: 7.2 T: 2°CAssay Description:Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T...More data for this Ligand-Target Pair
Affinity DataIC50: 53nMpH: 7.2 T: 2°CAssay Description:Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+3nMpH: 7.2 T: 2°CAssay Description:Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T...More data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMpH: 7.2 T: 2°CAssay Description:Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+3nMpH: 7.2 T: 2°CAssay Description:Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T...More data for this Ligand-Target Pair

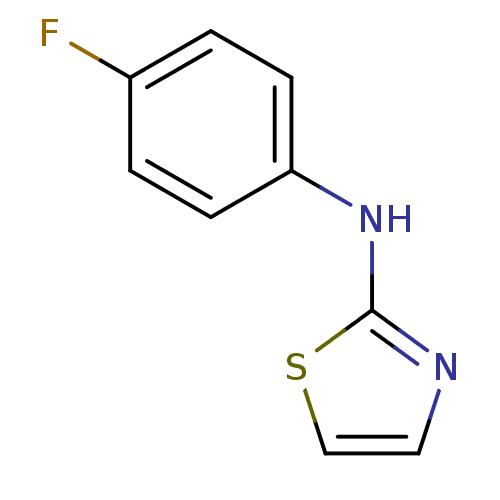


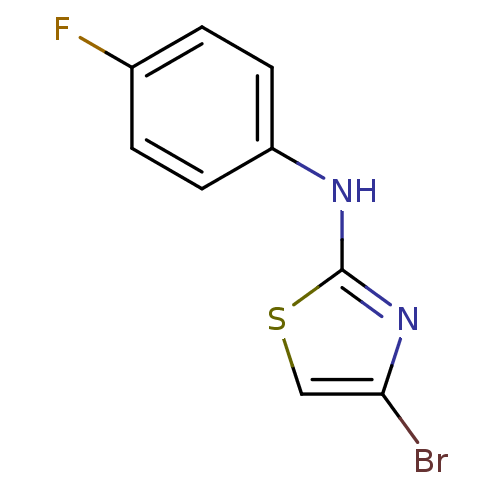

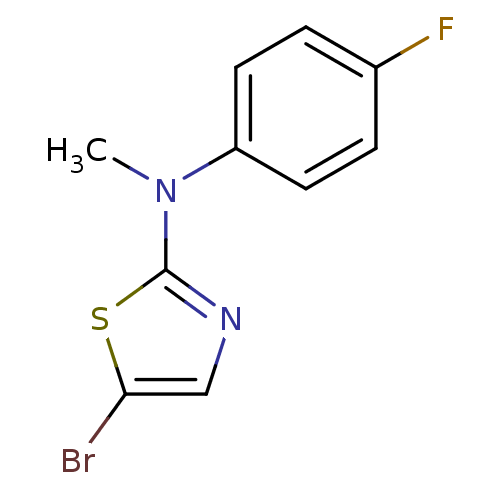
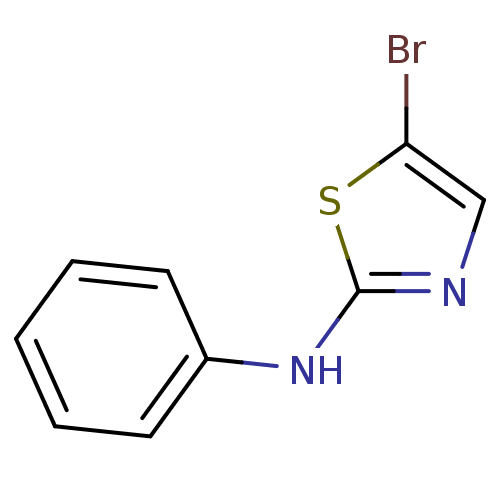
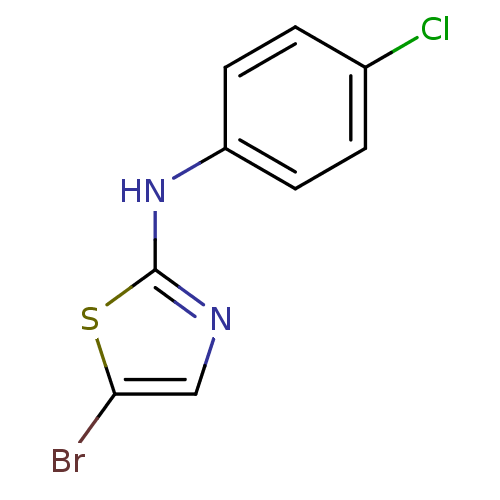

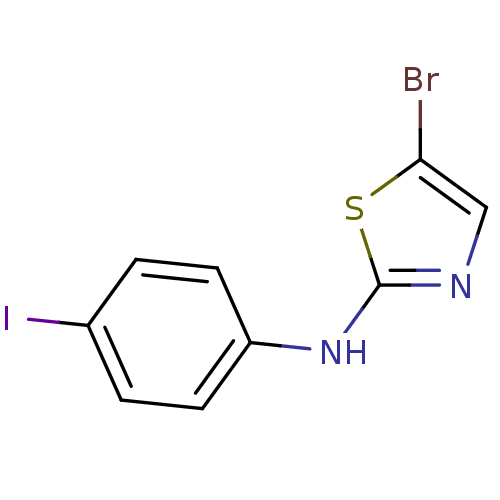






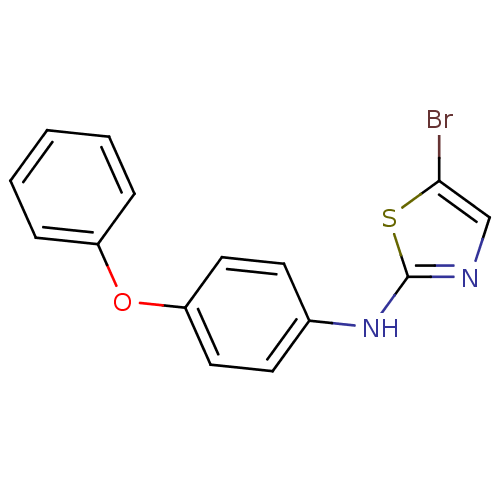


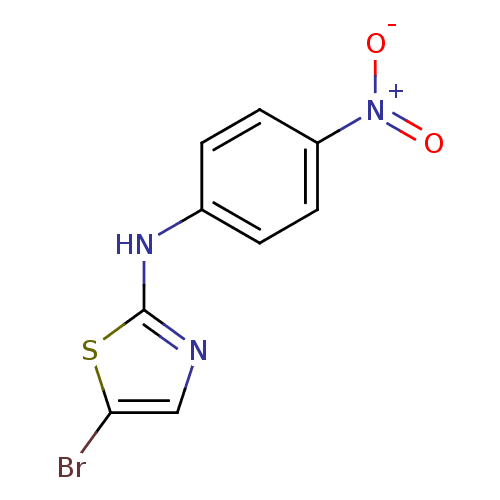

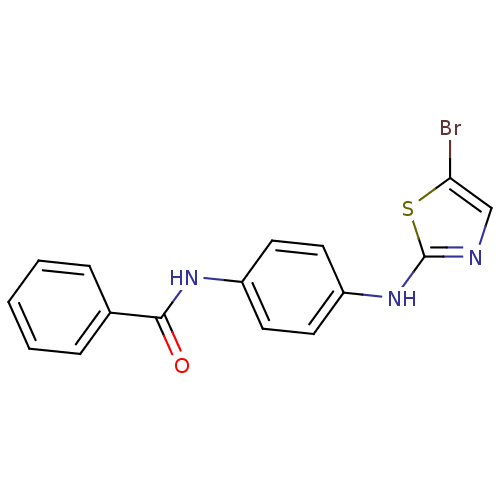
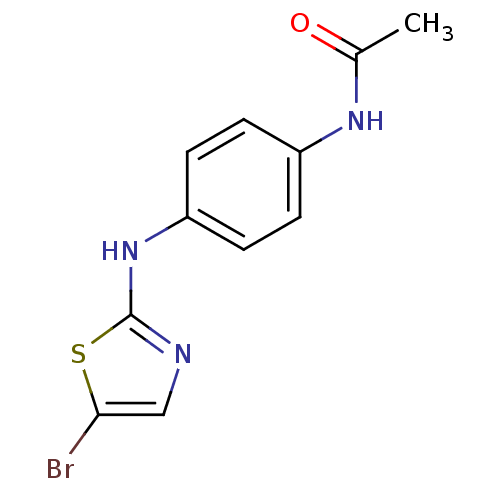




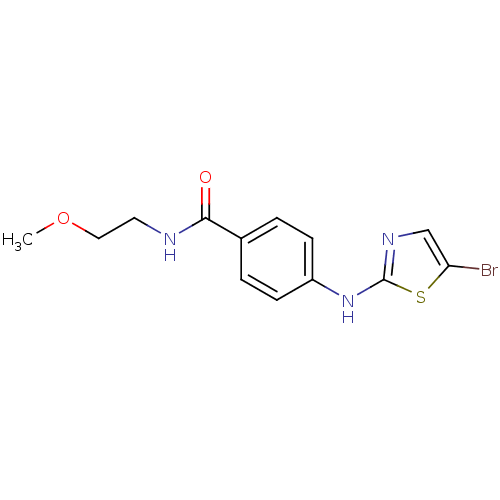

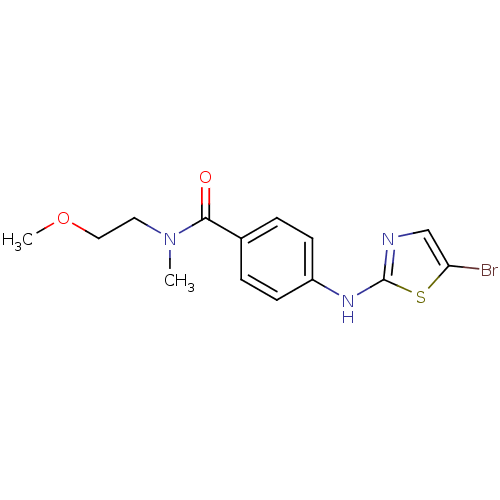
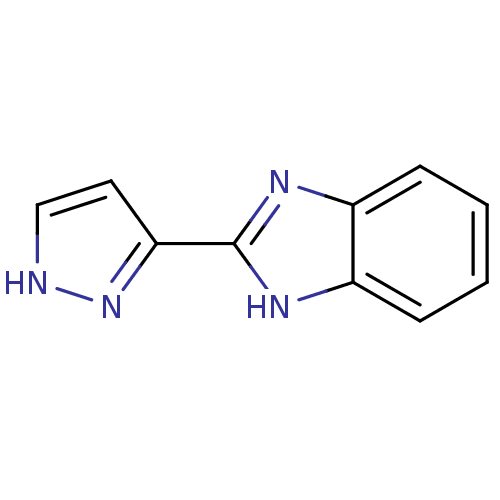
 3D Structure (crystal)
3D Structure (crystal)