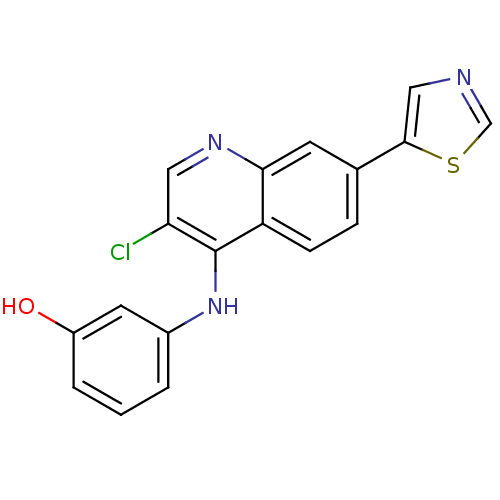
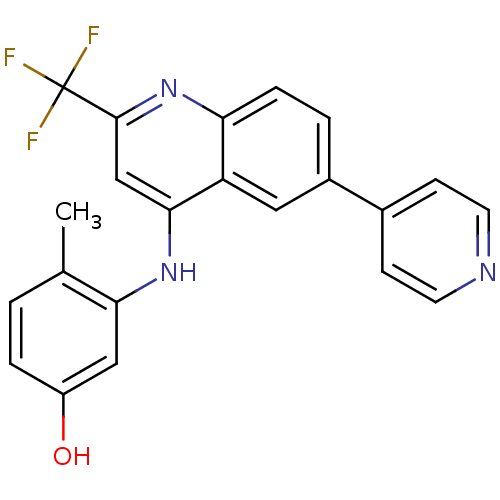
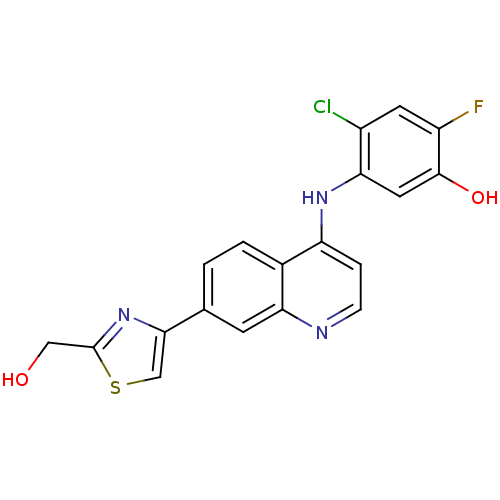
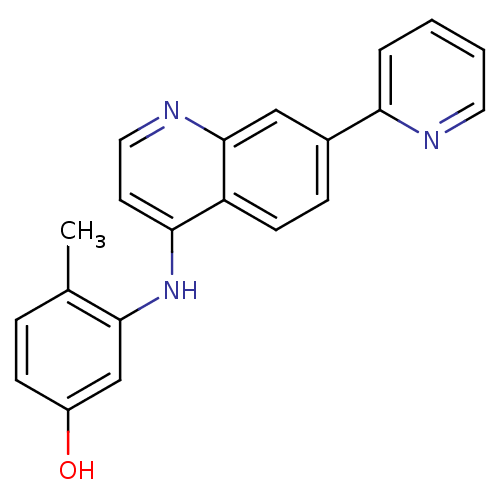
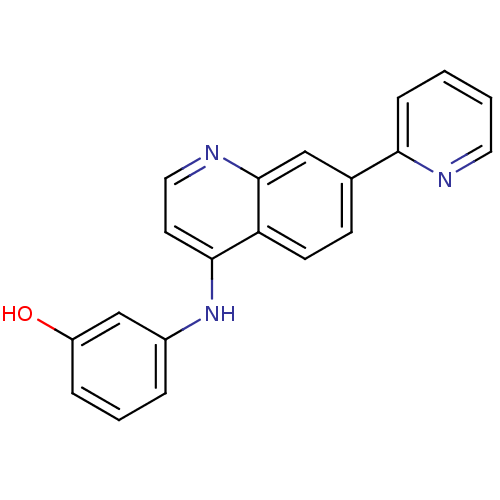
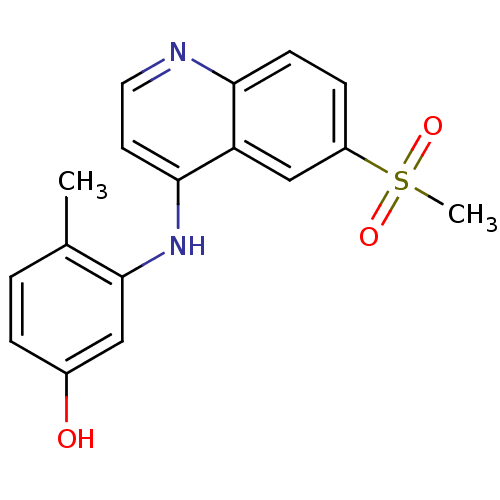
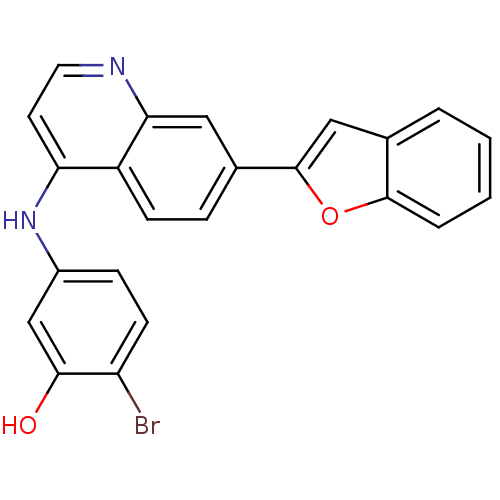
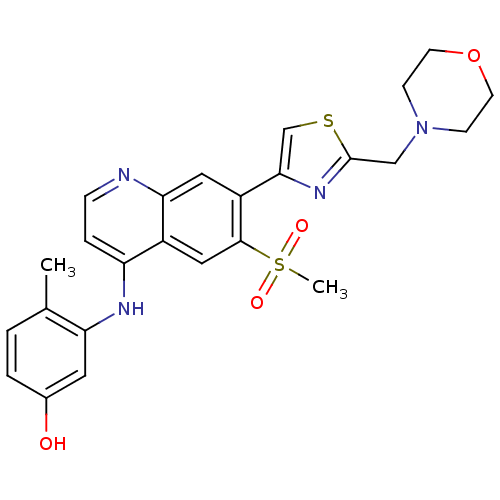
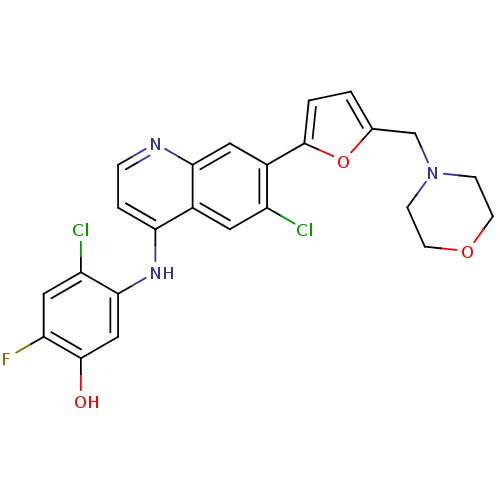
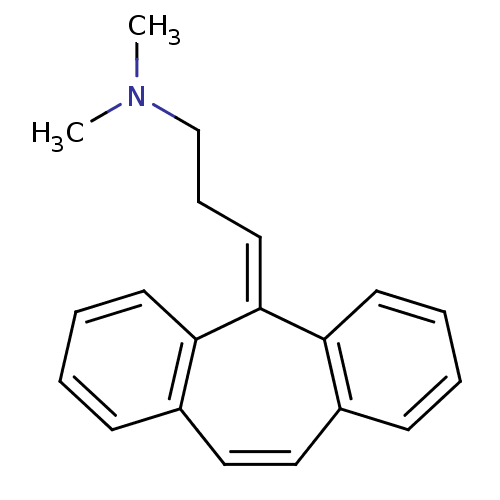
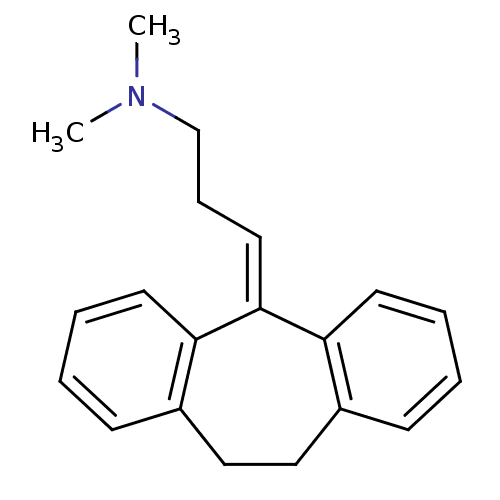
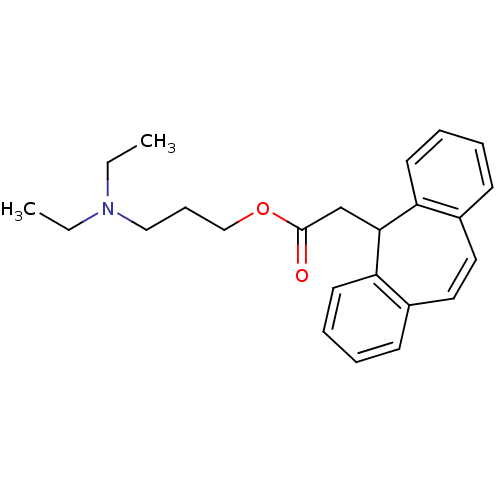
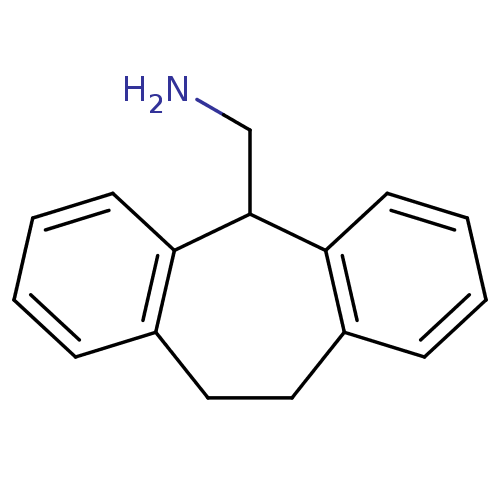
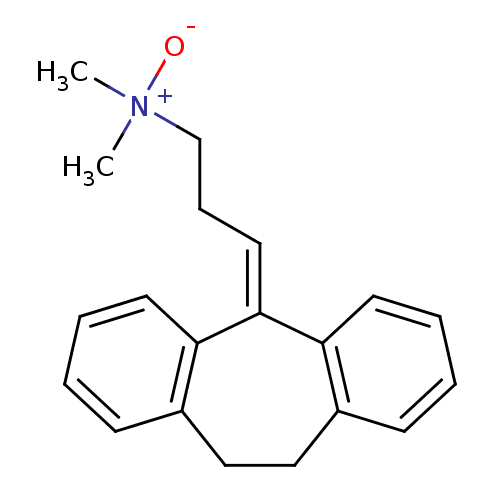
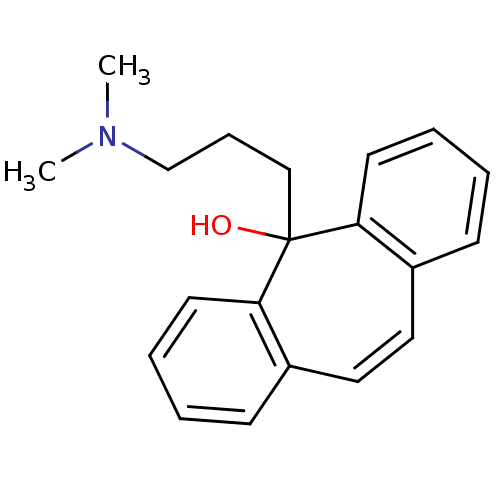
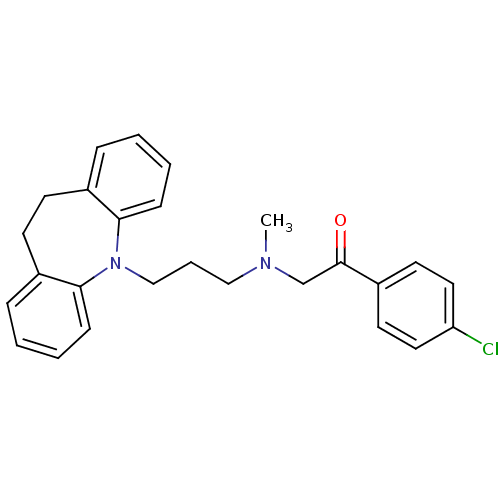
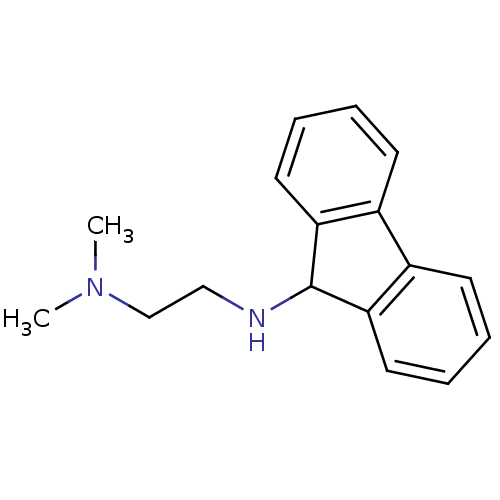
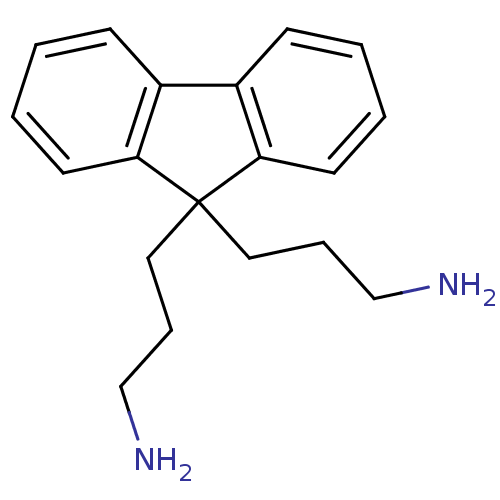
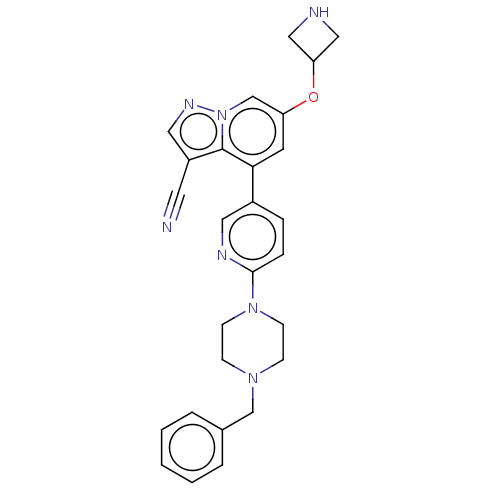
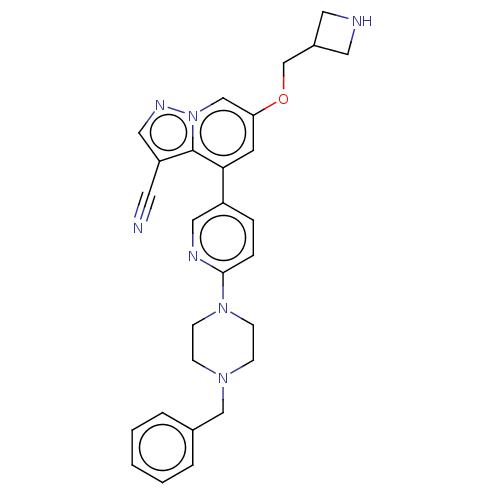
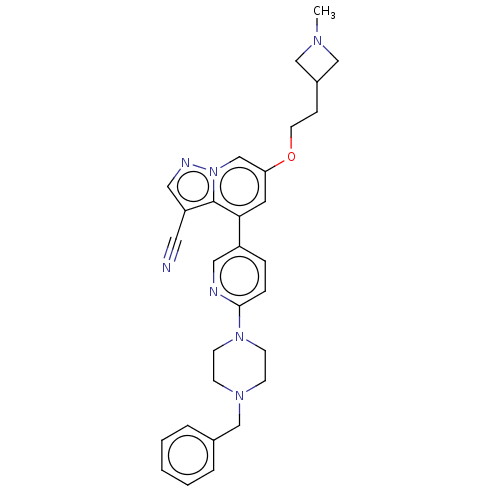
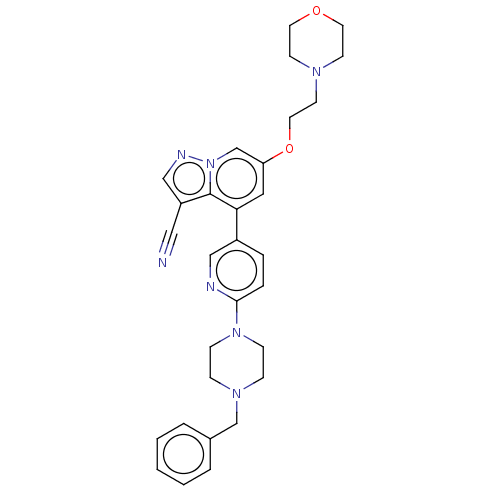
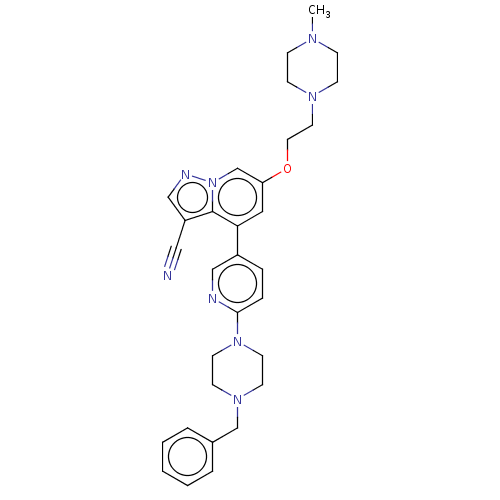
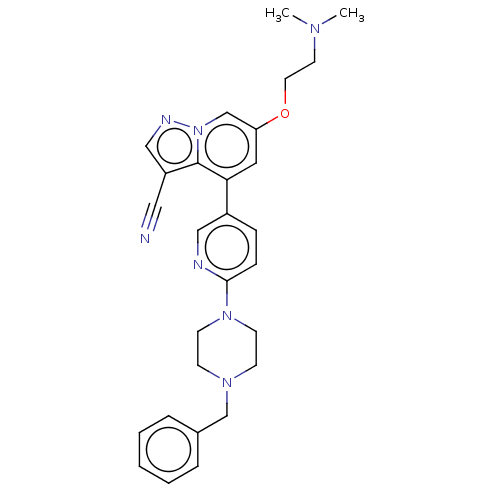
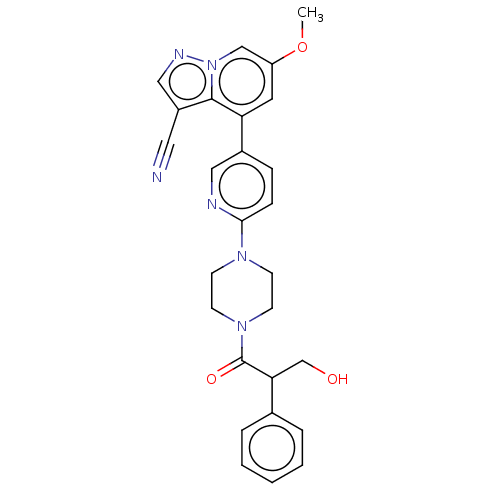
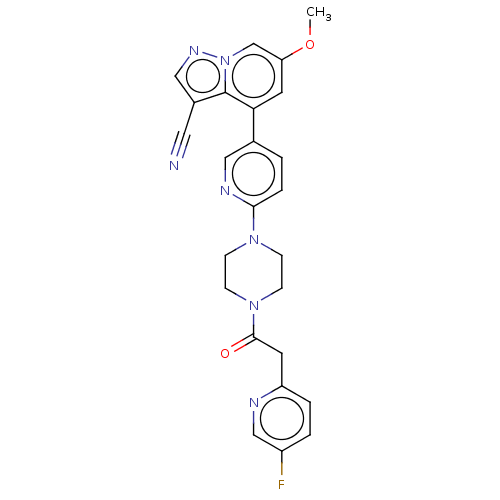
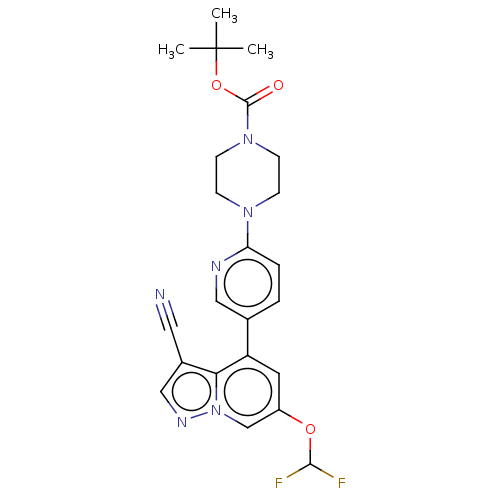
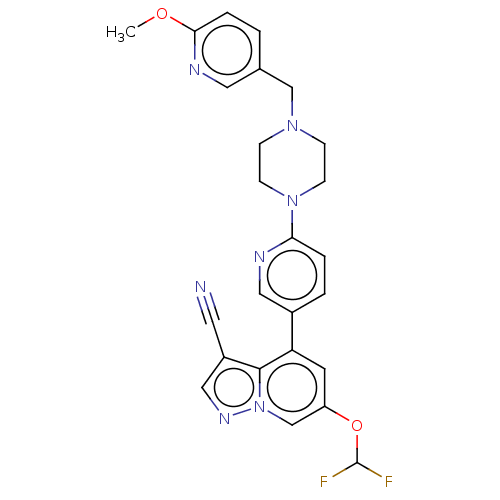
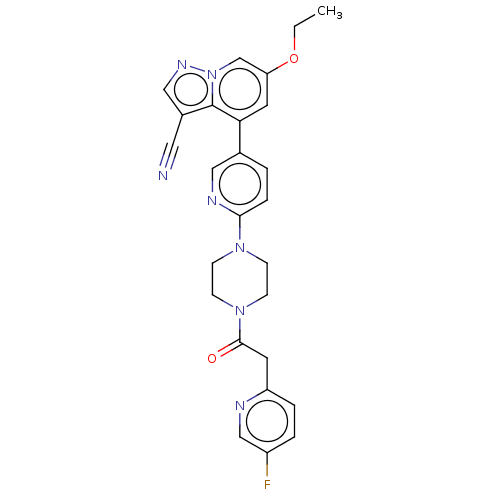
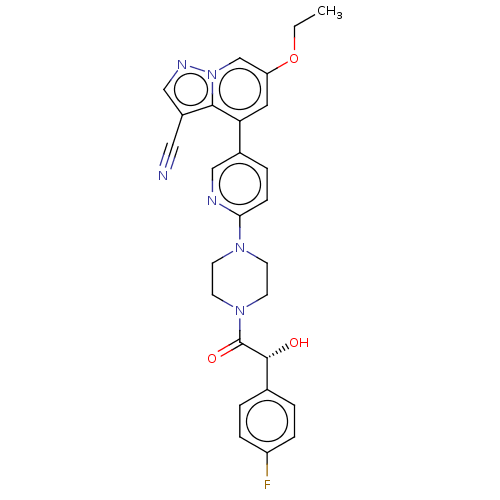
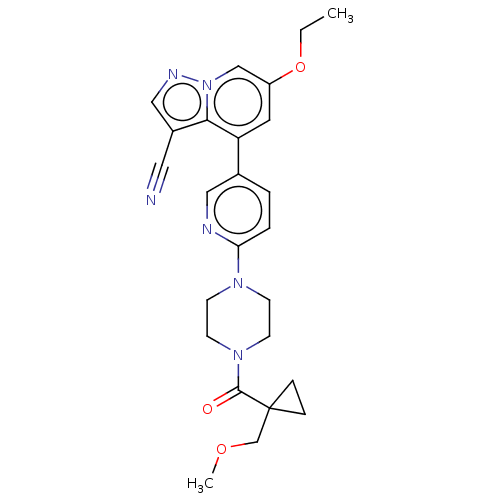
Affinity DataIC50: 100nMpH: 7.4 T: 2°CAssay Description:The biochemical activity of compound was determined by incubation with Ret kinase and the substrate in the presence of ATP/ [gamma-33P] ATP. After in...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+3nMpH: 7.4 T: 2°CAssay Description:The biochemical activity of compound was determined by incubation with Ret kinase and the substrate in the presence of ATP/ [gamma-33P] ATP. After in...More data for this Ligand-Target Pair
Affinity DataIC50: 75nMpH: 7.4 T: 2°CAssay Description:The biochemical activity of compound was determined by incubation with Ret kinase and the substrate in the presence of ATP/ [gamma-33P] ATP. After in...More data for this Ligand-Target Pair
Affinity DataIC50: 25nMpH: 7.4 T: 2°CAssay Description:The biochemical activity of compound was determined by incubation with Ret kinase and the substrate in the presence of ATP/ [gamma-33P] ATP. After in...More data for this Ligand-Target Pair
Affinity DataIC50: 25nMpH: 7.4 T: 2°CAssay Description:The biochemical activity of compound was determined by incubation with Ret kinase and the substrate in the presence of ATP/ [gamma-33P] ATP. After in...More data for this Ligand-Target Pair
Affinity DataIC50: 75nMpH: 7.4 T: 2°CAssay Description:The biochemical activity of compound was determined by incubation with Ret kinase and the substrate in the presence of ATP/ [gamma-33P] ATP. After in...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMpH: 7.4 T: 2°CAssay Description:The biochemical activity of compound was determined by incubation with Ret kinase and the substrate in the presence of ATP/ [gamma-33P] ATP. After in...More data for this Ligand-Target Pair
Affinity DataIC50: 80nMpH: 7.4 T: 2°CAssay Description:The biochemical activity of compound was determined by incubation with Ret kinase and the substrate in the presence of ATP/ [gamma-33P] ATP. After in...More data for this Ligand-Target Pair
Affinity DataIC50: 95nMpH: 7.4 T: 2°CAssay Description:The biochemical activity of compound was determined by incubation with Ret kinase and the substrate in the presence of ATP/ [gamma-33P] ATP. After in...More data for this Ligand-Target Pair
Affinity DataIC50: 90nMpH: 7.4 T: 2°CAssay Description:The biochemical activity of compound was determined by incubation with Ret kinase and the substrate in the presence of ATP/ [gamma-33P] ATP. After in...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMpH: 7.4 T: 2°CAssay Description:The biochemical activity of compound was determined by incubation with Ret kinase and the substrate in the presence of ATP/ [gamma-33P] ATP. After in...More data for this Ligand-Target Pair
Affinity DataIC50: 85nMpH: 7.4 T: 2°CAssay Description:The biochemical activity of compound was determined by incubation with Ret kinase and the substrate in the presence of ATP/ [gamma-33P] ATP. After in...More data for this Ligand-Target Pair
Affinity DataIC50: 34nMpH: 7.4 T: 2°CAssay Description:The biochemical activity of compound was determined by incubation with Ret kinase and the substrate in the presence of ATP/ [gamma-33P] ATP. After in...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+3nMpH: 7.4 T: 2°CAssay Description:The biochemical activity of compound was determined by incubation with Ret kinase and the substrate in the presence of ATP/ [gamma-33P] ATP. After in...More data for this Ligand-Target Pair
Affinity DataIC50: 300nMpH: 7.4 T: 2°CAssay Description:Approximately 5-20 μg of purified GST-RET proteins were incubated with 1 mM ATP in 20 μL kinase buffer (10 mM Tris-HCl, 5 mM MgCl2, pH 7.4)...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMpH: 7.4 T: 2°CAssay Description:Approximately 5-20 μg of purified GST-RET proteins were incubated with 1 mM ATP in 20 μL kinase buffer (10 mM Tris-HCl, 5 mM MgCl2, pH 7.4)...More data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+3nMpH: 7.4 T: 2°CAssay Description:Approximately 5-20 μg of purified GST-RET proteins were incubated with 1 mM ATP in 20 μL kinase buffer (10 mM Tris-HCl, 5 mM MgCl2, pH 7.4)...More data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+3nMpH: 7.4 T: 2°CAssay Description:Approximately 5-20 μg of purified GST-RET proteins were incubated with 1 mM ATP in 20 μL kinase buffer (10 mM Tris-HCl, 5 mM MgCl2, pH 7.4)...More data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+3nMpH: 7.4 T: 2°CAssay Description:Approximately 5-20 μg of purified GST-RET proteins were incubated with 1 mM ATP in 20 μL kinase buffer (10 mM Tris-HCl, 5 mM MgCl2, pH 7.4)...More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+3nMpH: 7.4 T: 2°CAssay Description:Approximately 5-20 μg of purified GST-RET proteins were incubated with 1 mM ATP in 20 μL kinase buffer (10 mM Tris-HCl, 5 mM MgCl2, pH 7.4)...More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+3nMpH: 7.4 T: 2°CAssay Description:Approximately 5-20 μg of purified GST-RET proteins were incubated with 1 mM ATP in 20 μL kinase buffer (10 mM Tris-HCl, 5 mM MgCl2, pH 7.4)...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMpH: 7.4 T: 2°CAssay Description:Approximately 5-20 μg of purified GST-RET proteins were incubated with 1 mM ATP in 20 μL kinase buffer (10 mM Tris-HCl, 5 mM MgCl2, pH 7.4)...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMpH: 7.4 T: 2°CAssay Description:Approximately 5-20 μg of purified GST-RET proteins were incubated with 1 mM ATP in 20 μL kinase buffer (10 mM Tris-HCl, 5 mM MgCl2, pH 7.4)...More data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMpH: 7.4 T: 2°CAssay Description:Approximately 5-20 μg of purified GST-RET proteins were incubated with 1 mM ATP in 20 μL kinase buffer (10 mM Tris-HCl, 5 mM MgCl2, pH 7.4)...More data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMpH: 7.4 T: 2°CAssay Description:Approximately 5-20 μg of purified GST-RET proteins were incubated with 1 mM ATP in 20 μL kinase buffer (10 mM Tris-HCl, 5 mM MgCl2, pH 7.4)...More data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMpH: 7.4 T: 2°CAssay Description:Approximately 5-20 μg of purified GST-RET proteins were incubated with 1 mM ATP in 20 μL kinase buffer (10 mM Tris-HCl, 5 mM MgCl2, pH 7.4)...More data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMpH: 7.4 T: 2°CAssay Description:Approximately 5-20 μg of purified GST-RET proteins were incubated with 1 mM ATP in 20 μL kinase buffer (10 mM Tris-HCl, 5 mM MgCl2, pH 7.4)...More data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMpH: 7.4 T: 2°CAssay Description:Approximately 5-20 μg of purified GST-RET proteins were incubated with 1 mM ATP in 20 μL kinase buffer (10 mM Tris-HCl, 5 mM MgCl2, pH 7.4)...More data for this Ligand-Target Pair
Affinity DataIC50: 165nMpH: 7.4Assay Description:Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF® KinEASE¿-TK assay tec...More data for this Ligand-Target Pair
Affinity DataIC50: 264nMpH: 7.4Assay Description:Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF® KinEASE¿-TK assay tec...More data for this Ligand-Target Pair
Affinity DataIC50: 14.1nMpH: 7.4Assay Description:Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF® KinEASE¿-TK assay tec...More data for this Ligand-Target Pair
Affinity DataIC50: 18.1nMpH: 7.4Assay Description:Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF® KinEASE¿-TK assay tec...More data for this Ligand-Target Pair
Affinity DataIC50: 11.7nMpH: 7.4Assay Description:Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF® KinEASE¿-TK assay tec...More data for this Ligand-Target Pair
Affinity DataIC50: 11.4nMpH: 7.4Assay Description:Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF® KinEASE¿-TK assay tec...More data for this Ligand-Target Pair
Affinity DataIC50: 30.9nMpH: 7.4Assay Description:Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF® KinEASE¿-TK assay tec...More data for this Ligand-Target Pair
Affinity DataIC50: 20.2nMpH: 7.4Assay Description:Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF® KinEASE¿-TK assay tec...More data for this Ligand-Target Pair
Affinity DataIC50: 50.3nMpH: 7.4Assay Description:Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF® KinEASE¿-TK assay tec...More data for this Ligand-Target Pair
Affinity DataIC50: 39.9nMpH: 7.4Assay Description:Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF® KinEASE¿-TK assay tec...More data for this Ligand-Target Pair
Affinity DataIC50: 31nMpH: 7.4Assay Description:Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF® KinEASE¿-TK assay tec...More data for this Ligand-Target Pair
Affinity DataIC50: 259nMpH: 7.4Assay Description:Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF® KinEASE¿-TK assay tec...More data for this Ligand-Target Pair
Affinity DataIC50: 4.05E+3nMpH: 7.4Assay Description:Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF® KinEASE¿-TK assay tec...More data for this Ligand-Target Pair
Affinity DataIC50: 3.55E+3nMpH: 7.4Assay Description:Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF® KinEASE¿-TK assay tec...More data for this Ligand-Target Pair
Affinity DataIC50: 1.32E+3nMpH: 7.4Assay Description:Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF® KinEASE¿-TK assay tec...More data for this Ligand-Target Pair
Affinity DataIC50: 345nMpH: 7.4Assay Description:Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF® KinEASE¿-TK assay tec...More data for this Ligand-Target Pair
Affinity DataIC50: 434nMpH: 7.4Assay Description:Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF® KinEASE¿-TK assay tec...More data for this Ligand-Target Pair
Affinity DataIC50: 13.5nMpH: 7.4Assay Description:Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF® KinEASE¿-TK assay tec...More data for this Ligand-Target Pair
Affinity DataIC50: 69.6nMpH: 7.4Assay Description:Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF® KinEASE¿-TK assay tec...More data for this Ligand-Target Pair
Affinity DataIC50: 9.90nMpH: 7.4Assay Description:Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF® KinEASE¿-TK assay tec...More data for this Ligand-Target Pair
Affinity DataIC50: 19.7nMpH: 7.4Assay Description:Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF® KinEASE¿-TK assay tec...More data for this Ligand-Target Pair
Affinity DataIC50: 210nMpH: 7.4Assay Description:Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF® KinEASE¿-TK assay tec...More data for this Ligand-Target Pair