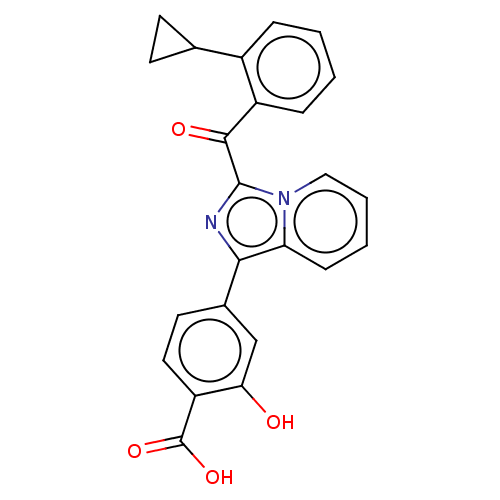
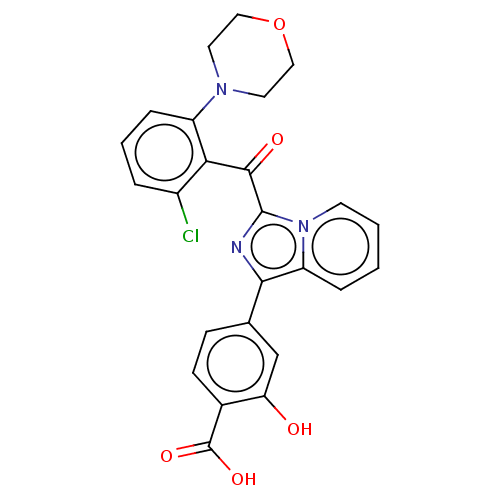
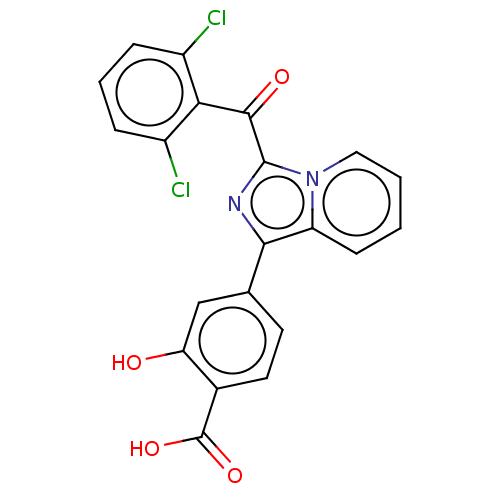
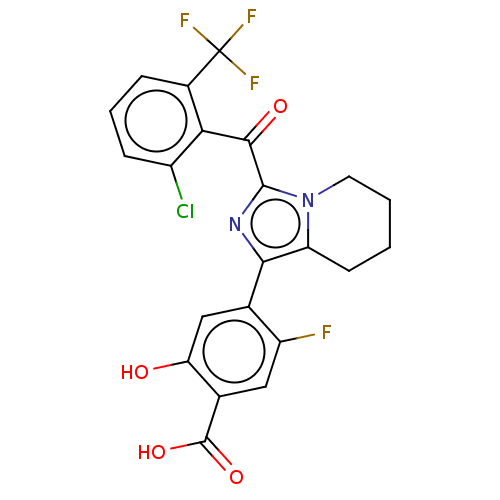
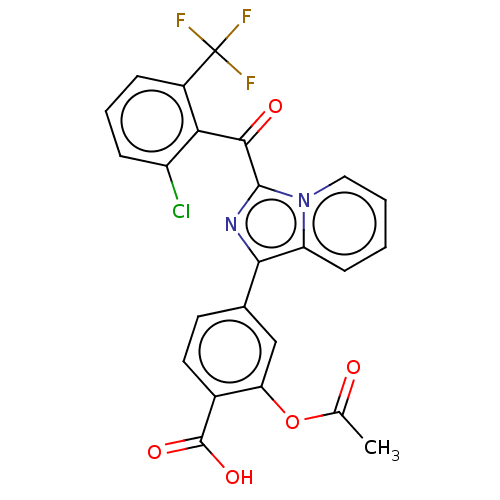
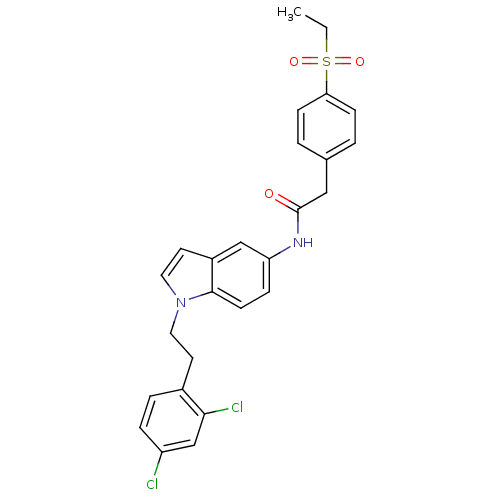
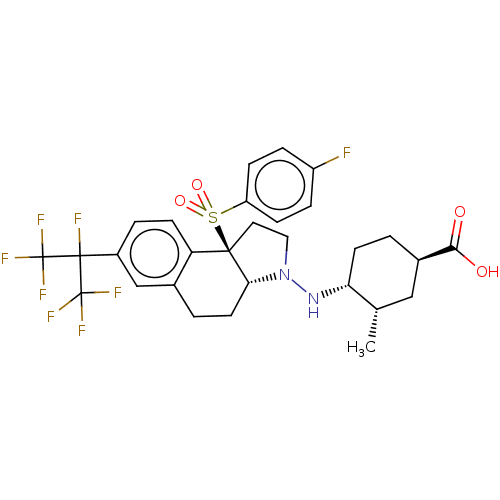
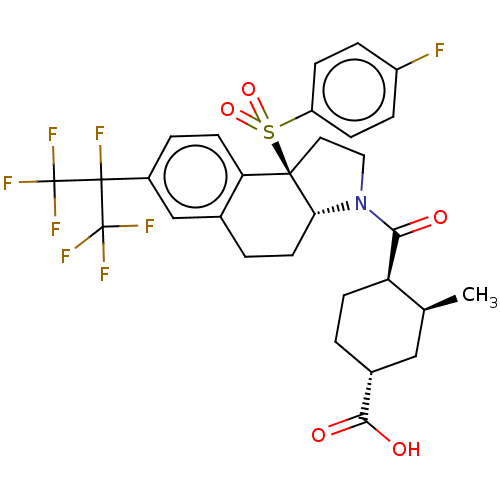
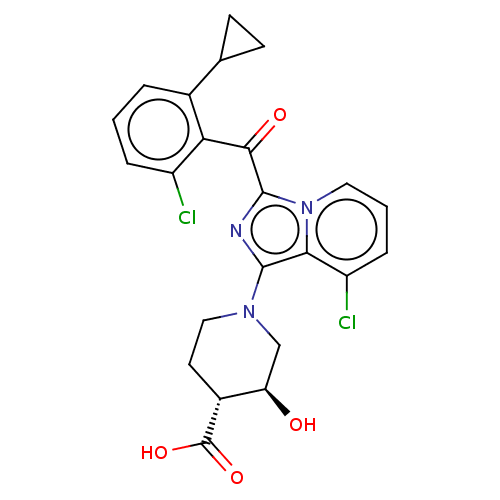
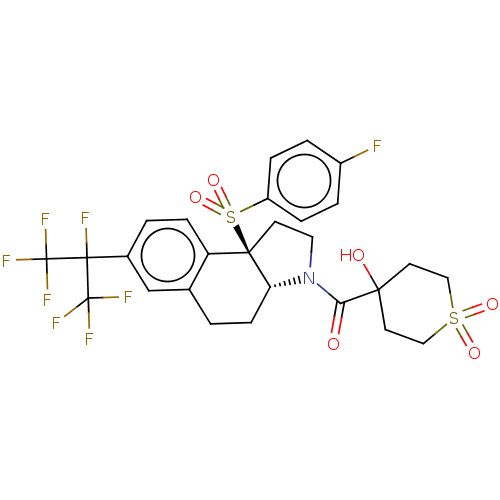
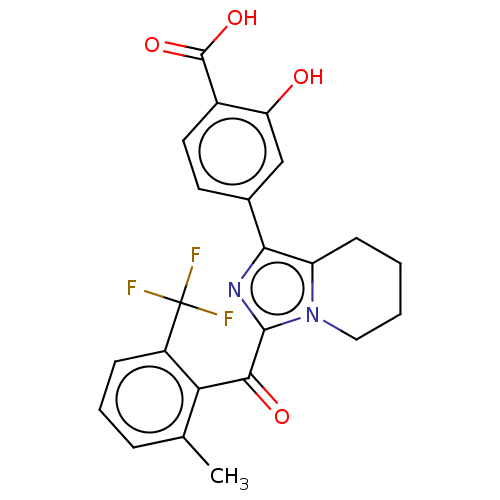
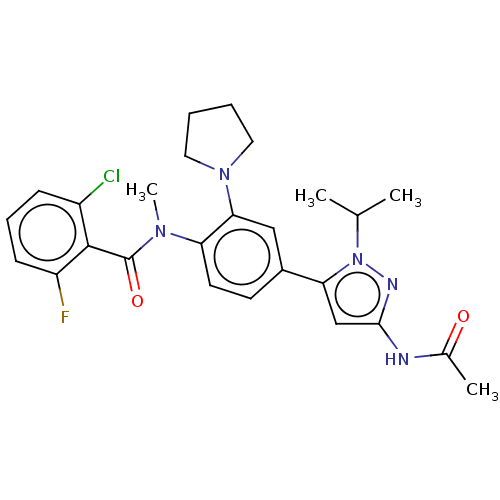
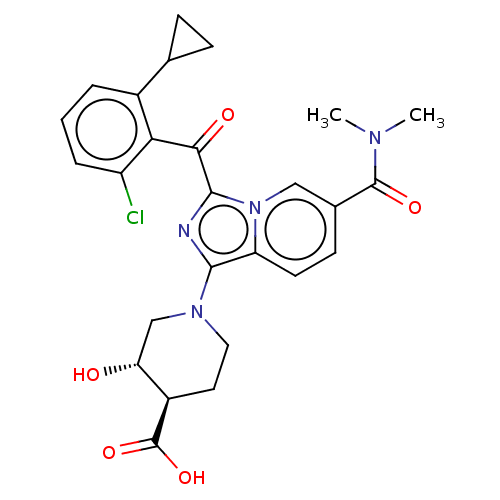
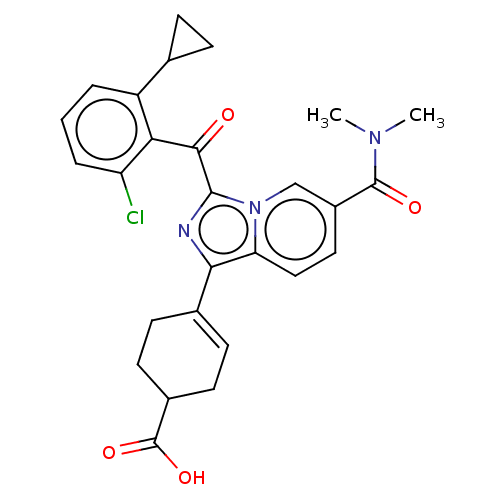
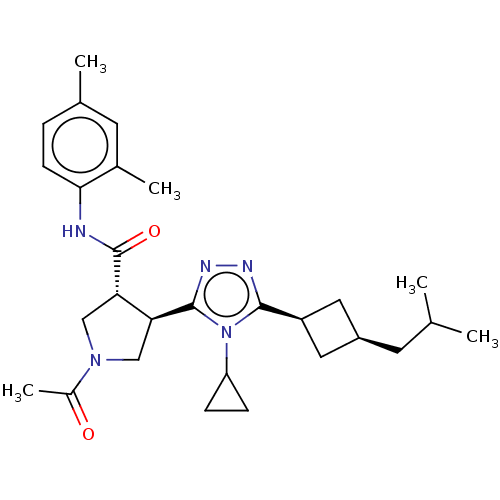
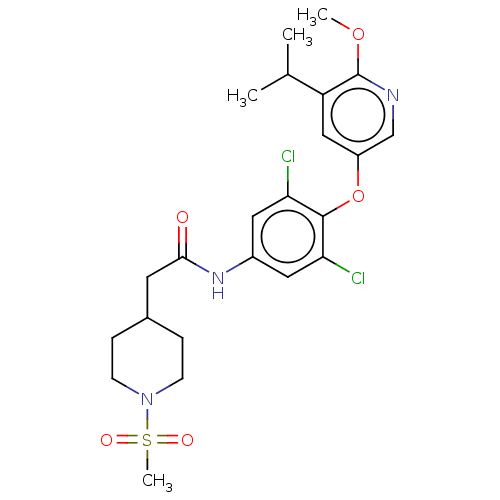
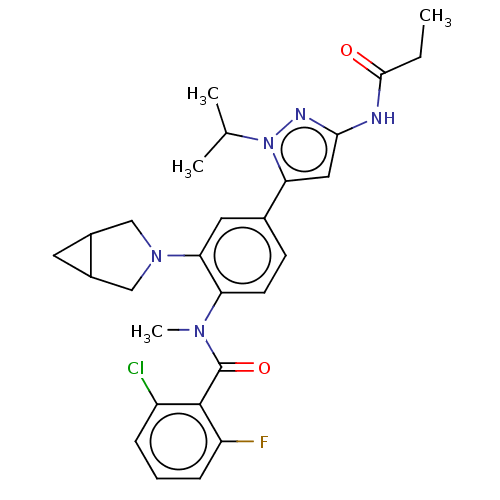
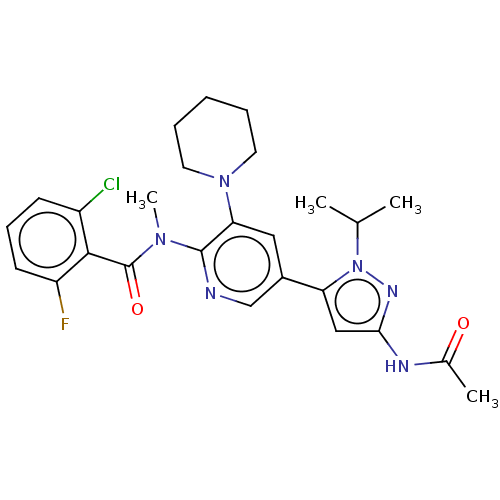
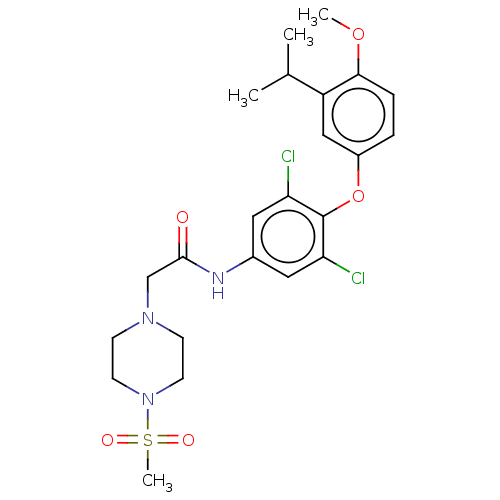
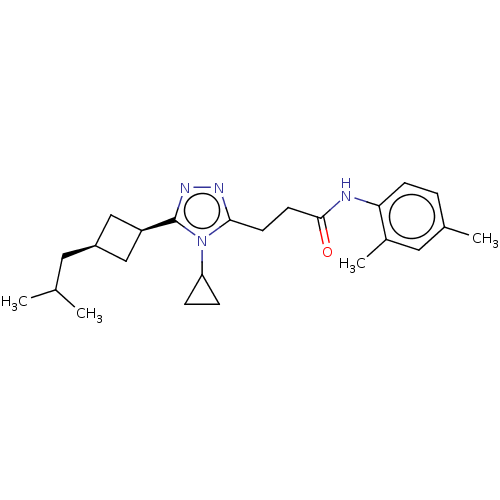
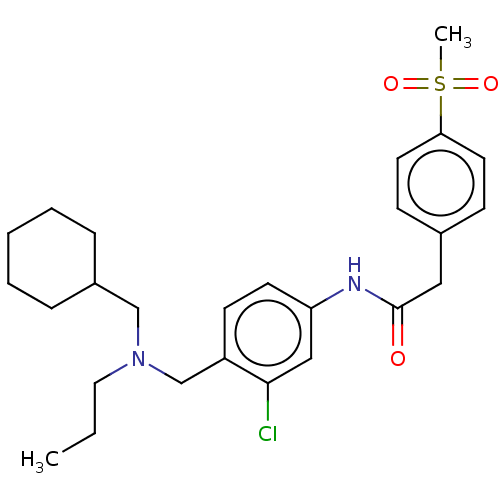
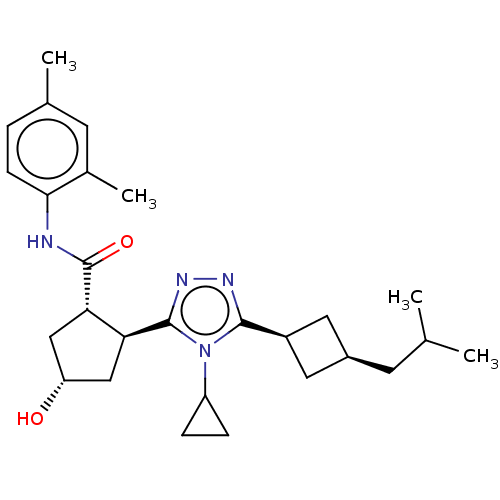
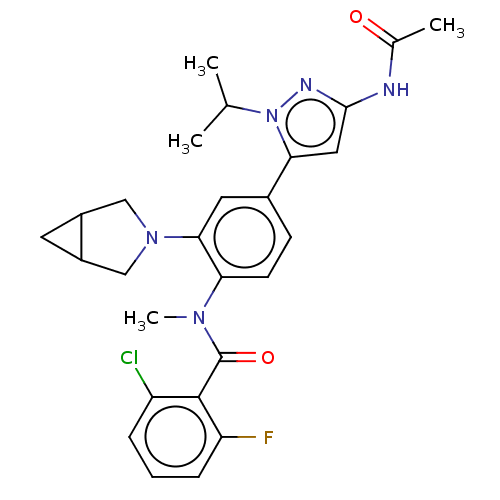
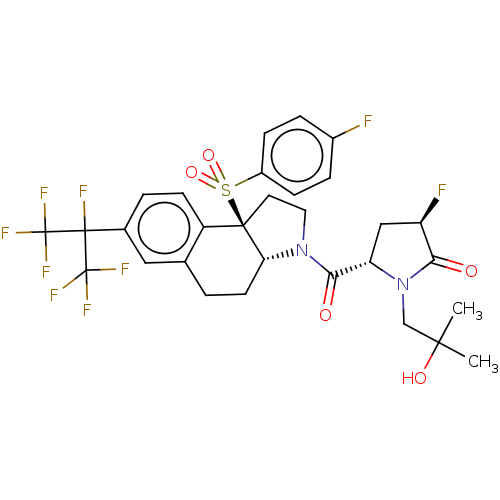
Affinity DataEC50: 2nMT: 2°CAssay Description:ssays were carried out in 16-μL reaction volumes in black 384 Plus F Proxiplates (Perkin-Elmer 6008269). All assay components except test ligand...More data for this Ligand-Target Pair
Affinity DataEC50: 2nMAssay Description:Inverse agonist activity at RORgammat in mouse Th17 cellsMore data for this Ligand-Target Pair
Affinity DataEC50: 2nMT: 2°CAssay Description:ssays were carried out in 16-μL reaction volumes in black 384 Plus F Proxiplates (Perkin-Elmer 6008269). All assay components except test ligand...More data for this Ligand-Target Pair
Affinity DataEC50: 2nMT: 2°CAssay Description:ssays were carried out in 16-μL reaction volumes in black 384 Plus F Proxiplates (Perkin-Elmer 6008269). All assay components except test ligand...More data for this Ligand-Target Pair
Affinity DataEC50: 2.70nMAssay Description:Inverse agonist activity at RORgammat in mouse Th17 cells assessed as inhibition of CD3/CD28/IL-1/IL-23-induced IL-17A production measured after 24 h...More data for this Ligand-Target Pair
Affinity DataEC50: 3nMT: 2°CAssay Description:ssays were carried out in 16-μL reaction volumes in black 384 Plus F Proxiplates (Perkin-Elmer 6008269). All assay components except test ligand...More data for this Ligand-Target Pair
Affinity DataEC50: 3nMT: 2°CAssay Description:ssays were carried out in 16-μL reaction volumes in black 384 Plus F Proxiplates (Perkin-Elmer 6008269). All assay components except test ligand...More data for this Ligand-Target Pair
Affinity DataEC50: 3nMT: 2°CAssay Description:ssays were carried out in 16-μL reaction volumes in black 384 Plus F Proxiplates (Perkin-Elmer 6008269). All assay components except test ligand...More data for this Ligand-Target Pair
Affinity DataEC50: 3nMT: 2°CAssay Description:ssays were carried out in 16-μL reaction volumes in black 384 Plus F Proxiplates (Perkin-Elmer 6008269). All assay components except test ligand...More data for this Ligand-Target Pair
Affinity DataEC50: 3nMT: 2°CAssay Description:ssays were carried out in 16-μL reaction volumes in black 384 Plus F Proxiplates (Perkin-Elmer 6008269). All assay components except test ligand...More data for this Ligand-Target Pair
Affinity DataEC50: 3nMT: 2°CAssay Description:ssays were carried out in 16-μL reaction volumes in black 384 Plus F Proxiplates (Perkin-Elmer 6008269). All assay components except test ligand...More data for this Ligand-Target Pair
Affinity DataEC50: 4nMT: 2°CAssay Description:ssays were carried out in 16-μL reaction volumes in black 384 Plus F Proxiplates (Perkin-Elmer 6008269). All assay components except test ligand...More data for this Ligand-Target Pair
Affinity DataEC50: 4nMT: 2°CAssay Description:ssays were carried out in 16-μL reaction volumes in black 384 Plus F Proxiplates (Perkin-Elmer 6008269). All assay components except test ligand...More data for this Ligand-Target Pair
Affinity DataEC50: 6nMT: 2°CAssay Description:ssays were carried out in 16-μL reaction volumes in black 384 Plus F Proxiplates (Perkin-Elmer 6008269). All assay components except test ligand...More data for this Ligand-Target Pair
Affinity DataEC50: 6nMT: 2°CAssay Description:ssays were carried out in 16-μL reaction volumes in black 384 Plus F Proxiplates (Perkin-Elmer 6008269). All assay components except test ligand...More data for this Ligand-Target Pair
Affinity DataEC50: 6nMT: 2°CAssay Description:ssays were carried out in 16-μL reaction volumes in black 384 Plus F Proxiplates (Perkin-Elmer 6008269). All assay components except test ligand...More data for this Ligand-Target Pair
Affinity DataEC50: 7nMT: 2°CAssay Description:ssays were carried out in 16-μL reaction volumes in black 384 Plus F Proxiplates (Perkin-Elmer 6008269). All assay components except test ligand...More data for this Ligand-Target Pair
Affinity DataEC50: 7nMT: 2°CAssay Description:ssays were carried out in 16-μL reaction volumes in black 384 Plus F Proxiplates (Perkin-Elmer 6008269). All assay components except test ligand...More data for this Ligand-Target Pair
Affinity DataEC50: 8nMT: 2°CAssay Description:ssays were carried out in 16-μL reaction volumes in black 384 Plus F Proxiplates (Perkin-Elmer 6008269). All assay components except test ligand...More data for this Ligand-Target Pair
Affinity DataEC50: 8nMT: 2°CAssay Description:ssays were carried out in 16-μL reaction volumes in black 384 Plus F Proxiplates (Perkin-Elmer 6008269). All assay components except test ligand...More data for this Ligand-Target Pair
Affinity DataEC50: 9nMT: 2°CAssay Description:ssays were carried out in 16-μL reaction volumes in black 384 Plus F Proxiplates (Perkin-Elmer 6008269). All assay components except test ligand...More data for this Ligand-Target Pair
Affinity DataEC50: 9nMT: 2°CAssay Description:ssays were carried out in 16-μL reaction volumes in black 384 Plus F Proxiplates (Perkin-Elmer 6008269). All assay components except test ligand...More data for this Ligand-Target Pair
Affinity DataEC50: 9nMAssay Description:Activity at RoRc in mouse CD4+ T cells assessed as inhibition of IL-17 productionMore data for this Ligand-Target Pair
Affinity DataEC50: 9nMT: 2°CAssay Description:ssays were carried out in 16-μL reaction volumes in black 384 Plus F Proxiplates (Perkin-Elmer 6008269). All assay components except test ligand...More data for this Ligand-Target Pair
Affinity DataEC50: 10nMT: 2°CAssay Description:ssays were carried out in 16-μL reaction volumes in black 384 Plus F Proxiplates (Perkin-Elmer 6008269). All assay components except test ligand...More data for this Ligand-Target Pair
Affinity DataEC50: 11nMAssay Description:Inverse agonist activity at RORgamma in C57BL/6 mouse splenocytes assessed as reduction of IL-17 production after 2 days by ELISAMore data for this Ligand-Target Pair
Affinity DataEC50: 11nMAssay Description:Inverse agonist activity at RORgammat in mouse Th17 cells assessed as inhibition of CD3/CD28/IL-1/IL-23-induced IL-17A production measured after 24 h...More data for this Ligand-Target Pair
Affinity DataEC50: 11nMAssay Description:Inverse agonist activity at RORgammat in mouse whole blood assessed as suppression of Th17 secretionMore data for this Ligand-Target Pair
Affinity DataEC50: 12nMT: 2°CAssay Description:ssays were carried out in 16-μL reaction volumes in black 384 Plus F Proxiplates (Perkin-Elmer 6008269). All assay components except test ligand...More data for this Ligand-Target Pair
Affinity DataEC50: 13nMT: 2°CAssay Description:ssays were carried out in 16-μL reaction volumes in black 384 Plus F Proxiplates (Perkin-Elmer 6008269). All assay components except test ligand...More data for this Ligand-Target Pair
Affinity DataEC50: 13nMAssay Description:Inverse agonist activity at RORgammat in mouse cells by mTh17 assayMore data for this Ligand-Target Pair
Affinity DataEC50: 14nMT: 2°CAssay Description:ssays were carried out in 16-μL reaction volumes in black 384 Plus F Proxiplates (Perkin-Elmer 6008269). All assay components except test ligand...More data for this Ligand-Target Pair
Affinity DataEC50: 17nMAssay Description:Inverse agonist activity at RORgamma in C57BL/6 mouse splenocytes assessed as reduction of IL-17 production after 2 days by ELISAMore data for this Ligand-Target Pair
Affinity DataEC50: 19nMT: 2°CAssay Description:ssays were carried out in 16-μL reaction volumes in black 384 Plus F Proxiplates (Perkin-Elmer 6008269). All assay components except test ligand...More data for this Ligand-Target Pair
Affinity DataEC50: 22nMT: 2°CAssay Description:ssays were carried out in 16-μL reaction volumes in black 384 Plus F Proxiplates (Perkin-Elmer 6008269). All assay components except test ligand...More data for this Ligand-Target Pair
Affinity DataEC50: 26nMT: 2°CAssay Description:ssays were carried out in 16-μL reaction volumes in black 384 Plus F Proxiplates (Perkin-Elmer 6008269). All assay components except test ligand...More data for this Ligand-Target Pair
Affinity DataEC50: 27nMT: 2°CAssay Description:ssays were carried out in 16-μL reaction volumes in black 384 Plus F Proxiplates (Perkin-Elmer 6008269). All assay components except test ligand...More data for this Ligand-Target Pair
Affinity DataEC50: 29nMAssay Description:Inhibition of mouse RORgamma ligand binding domain (Isoleucine 251 to Lysine 516) expressed in CHOK1 cells incubated for 2 days by Gal4 luciferase re...More data for this Ligand-Target Pair
Affinity DataEC50: 29nMT: 2°CAssay Description:ssays were carried out in 16-μL reaction volumes in black 384 Plus F Proxiplates (Perkin-Elmer 6008269). All assay components except test ligand...More data for this Ligand-Target Pair
Affinity DataEC50: 30nMAssay Description:Agonist activity at RORgammat in C57/B6 mouse Th17 cells assessed as increase in IL17A expression after 96 hrs by AlphaLISAMore data for this Ligand-Target Pair
Affinity DataEC50: 32nMT: 2°CAssay Description:ssays were carried out in 16-μL reaction volumes in black 384 Plus F Proxiplates (Perkin-Elmer 6008269). All assay components except test ligand...More data for this Ligand-Target Pair
Affinity DataEC50: 33nMAssay Description:Inverse agonist activity at RORgamma in C57BL/6 mouse splenocytes assessed as reduction of IL-17 production after 2 days by ELISAMore data for this Ligand-Target Pair
Affinity DataEC50: 34nMAssay Description:Inverse agonist activity at RORgamma in C57BL/6 mouse splenocytes assessed as reduction of IL-17 production after 2 days by ELISAMore data for this Ligand-Target Pair
Affinity DataEC50: 34nMAssay Description:Agonist activity at RORgammat in C57/B6 mouse Th17 cells assessed as increase in IL17A expression after 96 hrs by AlphaLISAMore data for this Ligand-Target Pair
Affinity DataEC50: 35nMAssay Description:Inhibition of mouse RORgamma ligand binding domain (Isoleucine 251 to Lysine 516) expressed in CHOK1 cells incubated for 2 days by Gal4 luciferase re...More data for this Ligand-Target Pair
Affinity DataEC50: 37nMAssay Description:Agonist activity at RORgammat in mouse CD4+ T cells assessed as induction of Th17 cells differentiation by measuring increase in IL17 production meas...More data for this Ligand-Target Pair
Affinity DataEC50: 38nMAssay Description:Inhibition of mouse RORgamma ligand binding domain (Isoleucine 251 to Lysine 516) expressed in CHOK1 cells incubated for 2 days by Gal4 luciferase re...More data for this Ligand-Target Pair
Affinity DataEC50: 38nMAssay Description:Inverse agonist activity at RORgamma in C57BL/6 mouse splenocytes assessed as reduction of IL-17 production after 2 days by ELISAMore data for this Ligand-Target Pair
Affinity DataEC50: 43nMAssay Description:Inverse agonist activity at RORgammat in mouse Th17 cellsMore data for this Ligand-Target Pair
Affinity DataEC50: 45nMT: 2°CAssay Description:ssays were carried out in 16-μL reaction volumes in black 384 Plus F Proxiplates (Perkin-Elmer 6008269). All assay components except test ligand...More data for this Ligand-Target Pair

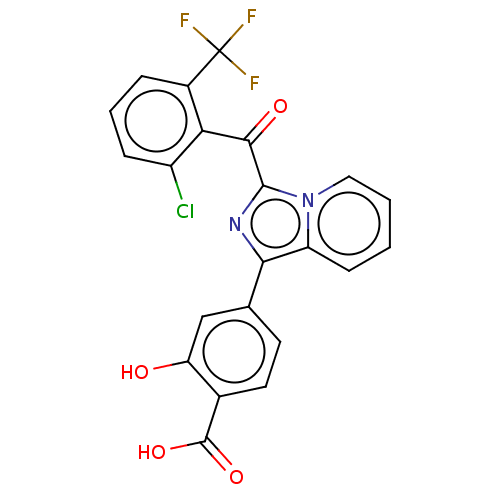
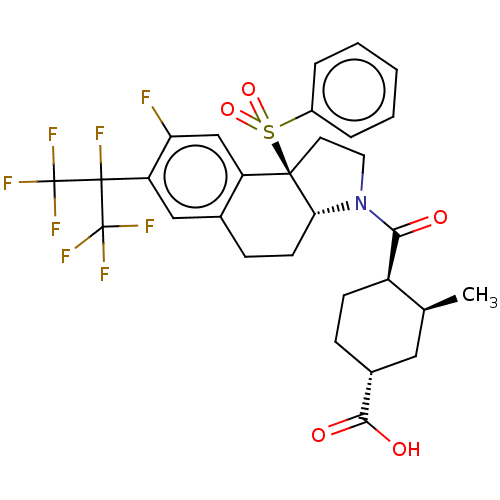

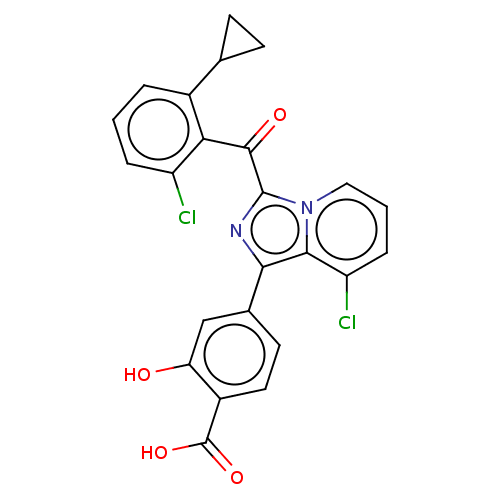
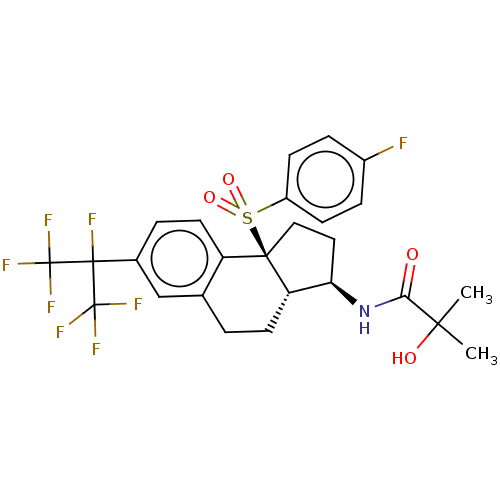
 3D Structure (crystal)
3D Structure (crystal)