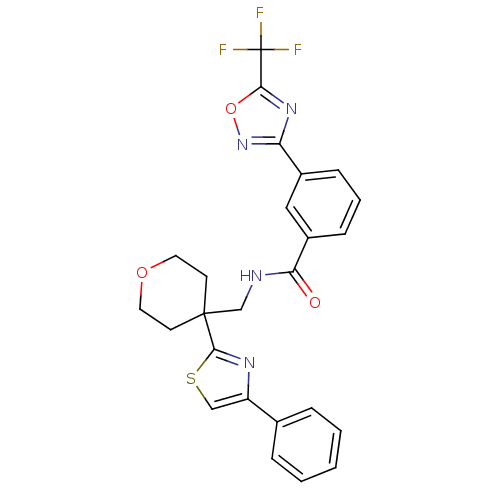
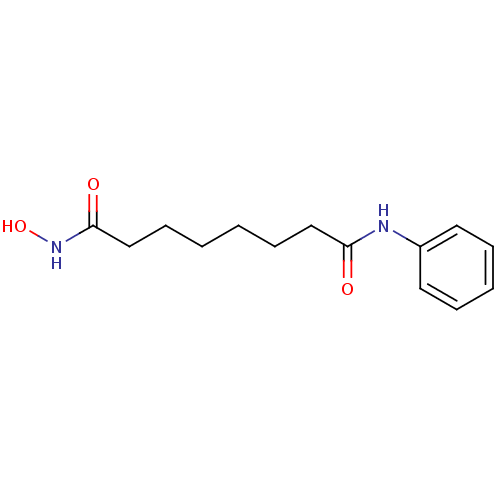
Affinity DataIC50: 0.958nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.05nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair
Affinity DataIC50: 10.3nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair
Affinity DataIC50: 12.9nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair
Affinity DataIC50: 17.7nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair
Affinity DataIC50: 20.7nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair
Affinity DataIC50: 23.9nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair
Affinity DataIC50: 27.1nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair
TargetHistone deacetylase 3/Nuclear receptor corepressor 2 [395-489](Homo sapiens (Human))
Reaction Biology
US Patent
Reaction Biology
US Patent
Affinity DataIC50: 29.9nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair
Affinity DataIC50: 32.4nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair
Affinity DataIC50: 65.7nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair
Affinity DataIC50: 175nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair
Affinity DataIC50: 213nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair
Affinity DataIC50: 293nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair
TargetHistone deacetylase 3/Nuclear receptor corepressor 2 [395-489](Homo sapiens (Human))
Reaction Biology
US Patent
Reaction Biology
US Patent
Affinity DataIC50: 435nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair
Affinity DataIC50: 472nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair
Affinity DataIC50: 508nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair
Affinity DataIC50: 597nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair
Affinity DataIC50: 604nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair
Affinity DataIC50: 683nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.13E+3nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair
TargetHistone deacetylase 3/Nuclear receptor corepressor 2 [395-489](Homo sapiens (Human))
Reaction Biology
US Patent
Reaction Biology
US Patent
Affinity DataIC50: 1.31E+3nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.41E+3nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.89E+3nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.84E+3nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.31E+3nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.53E+3nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair
Affinity DataIC50: 4.26E+3nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair
Affinity DataIC50: 4.29E+3nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.40E+3nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.78E+3nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair
Affinity DataIC50: 6.93E+3nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair
TargetHistone deacetylase 3/Nuclear receptor corepressor 2 [395-489](Homo sapiens (Human))
Reaction Biology
US Patent
Reaction Biology
US Patent
Affinity DataIC50: >1.00E+4nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.22E+4nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.74E+4nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair

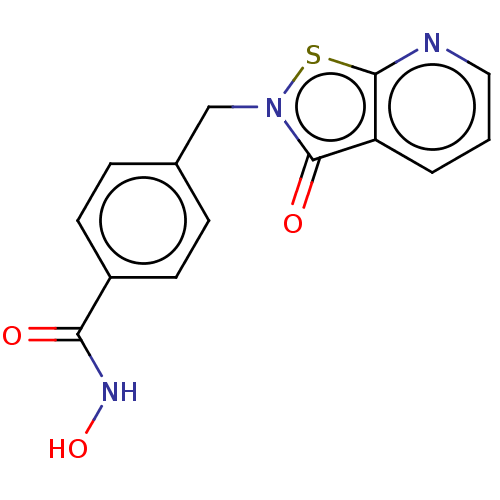
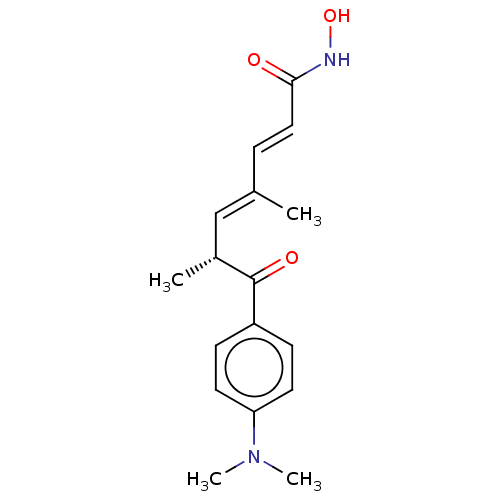
 3D Structure (crystal)
3D Structure (crystal)