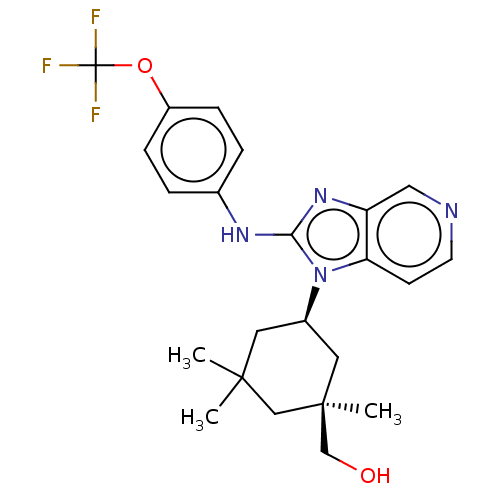
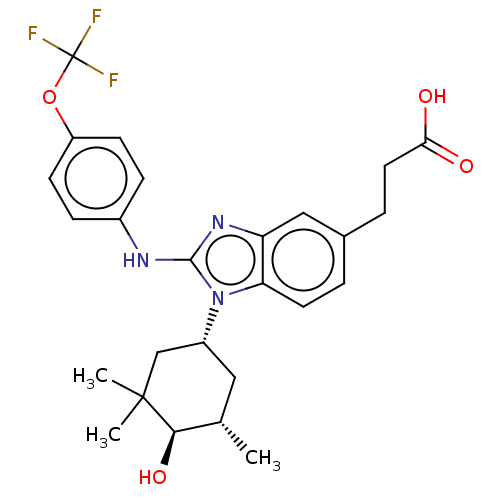
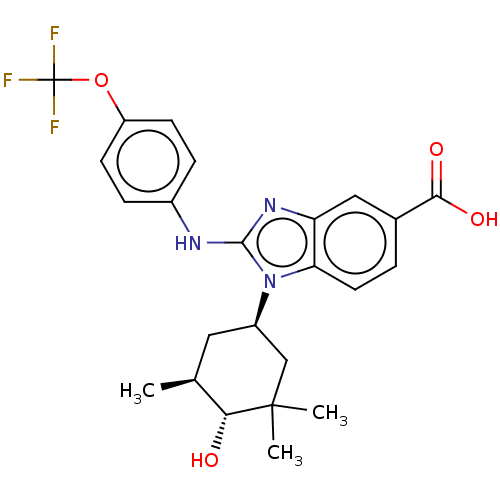
TargetIsocitrate dehydrogenase [NADP] cytoplasmic [R132H](Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 30nMAssay Description:mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by...More data for this Ligand-Target Pair
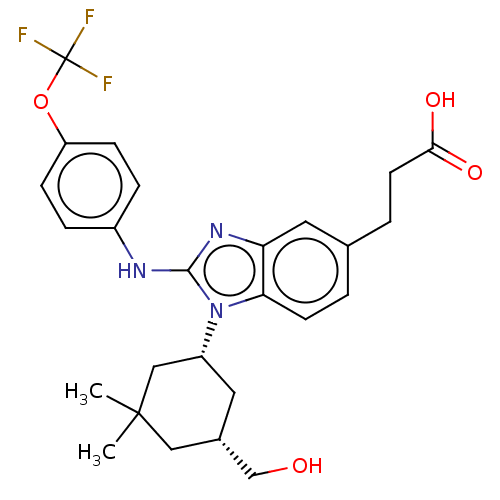
TargetIsocitrate dehydrogenase [NADP] cytoplasmic [R132H](Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 180nMAssay Description:mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by...More data for this Ligand-Target Pair
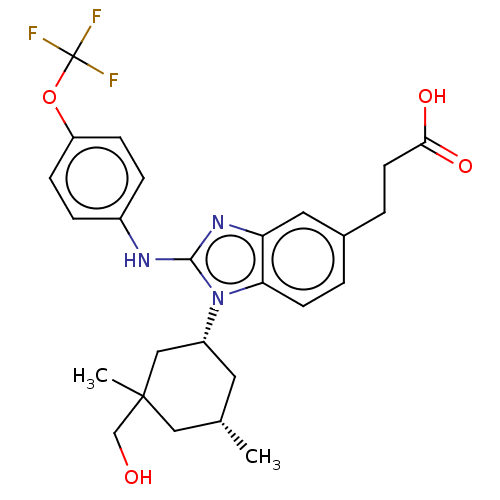
TargetIsocitrate dehydrogenase [NADP] cytoplasmic [R132H](Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 270nMAssay Description:mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by...More data for this Ligand-Target Pair
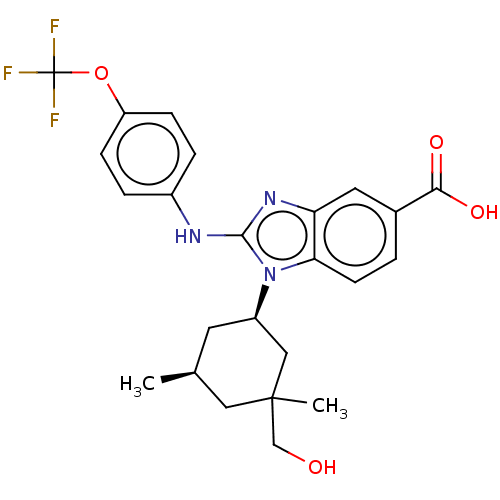
TargetIsocitrate dehydrogenase [NADP] cytoplasmic [R132H](Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 500nMAssay Description:mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by...More data for this Ligand-Target Pair
TargetIsocitrate dehydrogenase [NADP] cytoplasmic [R132H](Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 1.20E+3nMAssay Description:Levels of (2R)-2-hydroxyglutarate (2HG) were measured in medium of a cell line with overexpression of mutated isocitrate dehydrogenase (mIDH) protein...More data for this Ligand-Target Pair
TargetIsocitrate dehydrogenase [NADP] cytoplasmic [R132H](Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 1.60E+3nMAssay Description:Levels of (2R)-2-hydroxyglutarate (2HG) were measured in medium of a cell line with overexpression of mutated isocitrate dehydrogenase (mIDH) protein...More data for this Ligand-Target Pair
TargetIsocitrate dehydrogenase [NADP] cytoplasmic [R132H](Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 2.20E+3nMAssay Description:Levels of (2R)-2-hydroxyglutarate (2HG) were measured in medium of a cell line with overexpression of mutated isocitrate dehydrogenase (mIDH) protein...More data for this Ligand-Target Pair
TargetIsocitrate dehydrogenase [NADP] cytoplasmic [R132H](Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 4.10E+3nMAssay Description:mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by...More data for this Ligand-Target Pair
TargetIsocitrate dehydrogenase [NADP] cytoplasmic [R132H](Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 4.80E+3nMAssay Description:mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by...More data for this Ligand-Target Pair