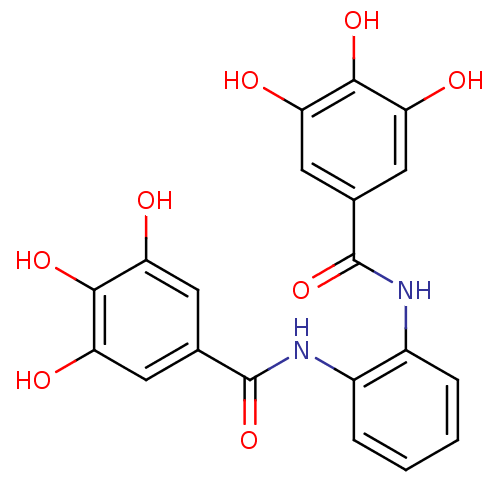
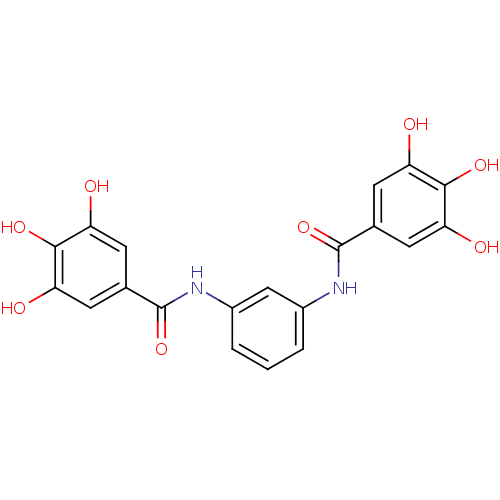
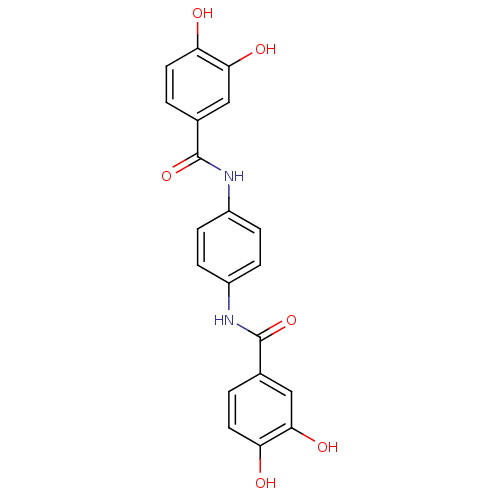
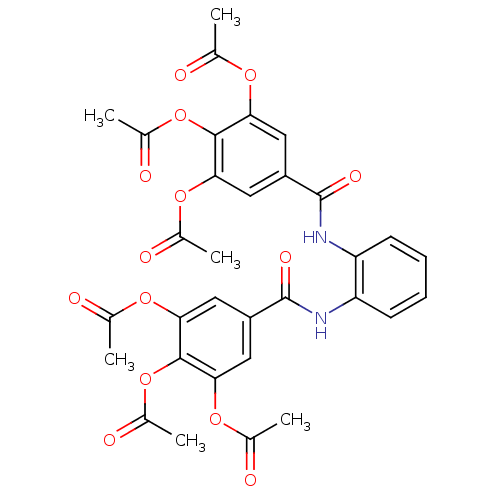
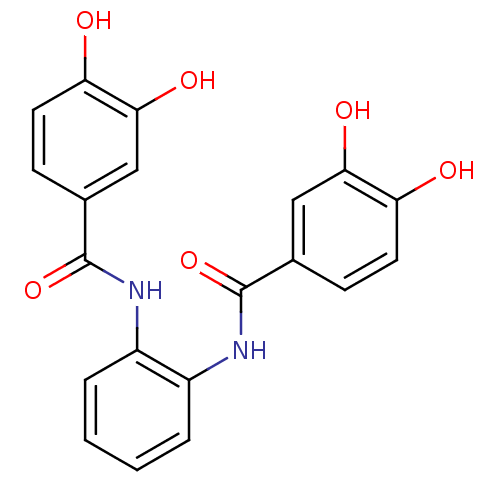
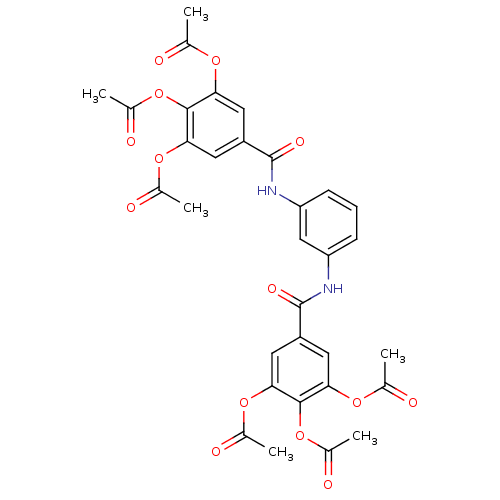
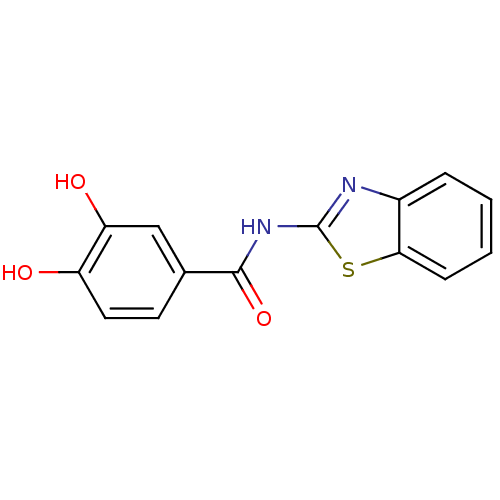
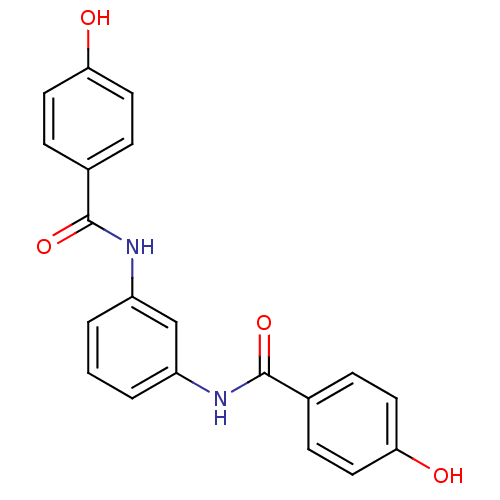
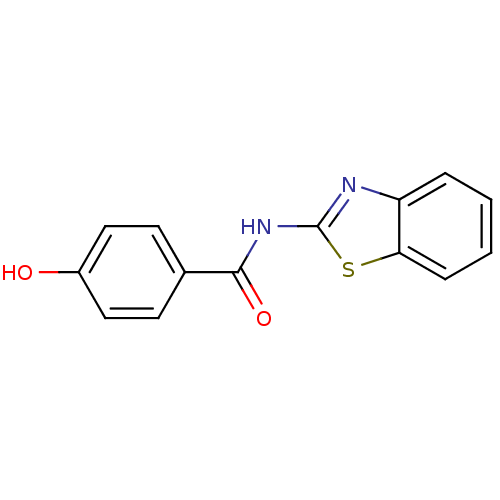
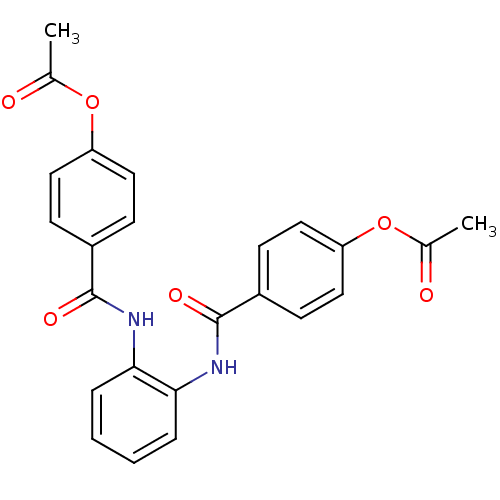
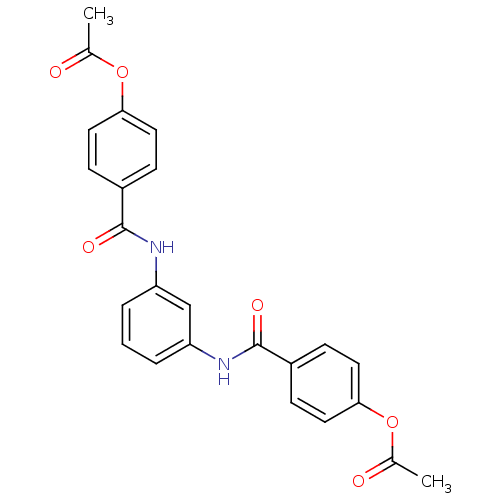
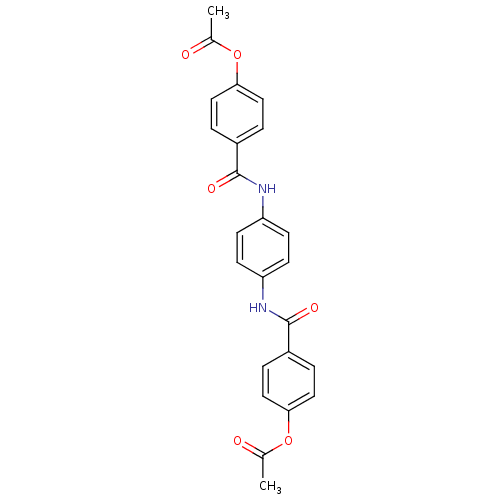
Affinity DataKi: 400nMAssay Description:Inhibition of relaxation activities of DNA topoisomerase I with respect to pBR322 DNAMore data for this Ligand-Target Pair
Affinity DataKi: 7.41E+4nMAssay Description:Inhibition of relaxation activities of DNA topoisomerase II with respect to pBR322 DNAMore data for this Ligand-Target Pair
Affinity DataIC50: 9nMAssay Description:Inhibitory concentration against relaxation activity of DNA topoisomerase II by detecting the conversion of supercoiled pBR322 DNA to its relaxed for...More data for this Ligand-Target Pair
Affinity DataIC50: 150nMAssay Description:Inhibitory concentration against relaxation activity of DNA topoisomerase II by detecting the conversion of supercoiled pBR322 DNA to its relaxed for...More data for this Ligand-Target Pair
Affinity DataIC50: 220nMAssay Description:Inhibitory concentration against relaxation activity of DNA topoisomerase II by detecting the conversion of supercoiled pBR322 DNA to its relaxed for...More data for this Ligand-Target Pair
Affinity DataIC50: 900nMAssay Description:Inhibitory concentration against relaxation activity of DNA topoisomerase I by detecting the conversion of supercoiled pBR322 DNA to its relaxed formMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40E+3nMAssay Description:Inhibitory concentration against relaxation activity of DNA topoisomerase I by detecting the conversion of supercoiled pBR322 DNA to its relaxed formMore data for this Ligand-Target Pair
Affinity DataIC50: 1.60E+3nMAssay Description:Inhibitory concentration against relaxation activity of DNA topoisomerase I by detecting the conversion of supercoiled pBR322 DNA to its relaxed formMore data for this Ligand-Target Pair
Affinity DataIC50: 2.60E+3nMAssay Description:Inhibitory concentration against relaxation activity of DNA topoisomerase I by detecting the conversion of supercoiled pBR322 DNA to its relaxed formMore data for this Ligand-Target Pair
Affinity DataIC50: 4.70E+3nMAssay Description:Inhibitory concentration against relaxation activity of DNA topoisomerase I by detecting the conversion of supercoiled pBR322 DNA to its relaxed formMore data for this Ligand-Target Pair
Affinity DataIC50: 6.40E+3nMAssay Description:Inhibitory concentration against relaxation activity of DNA topoisomerase I by detecting the conversion of supercoiled pBR322 DNA to its relaxed formMore data for this Ligand-Target Pair
Affinity DataIC50: 8.20E+3nMAssay Description:Inhibitory concentration against relaxation activity of DNA topoisomerase I by detecting the conversion of supercoiled pBR322 DNA to its relaxed formMore data for this Ligand-Target Pair
Affinity DataIC50: 8.60E+3nMAssay Description:Inhibitory concentration against relaxation activity of DNA topoisomerase I by detecting the conversion of supercoiled pBR322 DNA to its relaxed formMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibitory concentration against relaxation activity of DNA topoisomerase I by detecting the conversion of supercoiled pBR322 DNA to its relaxed formMore data for this Ligand-Target Pair
Affinity DataIC50: 1.12E+4nMAssay Description:Inhibitory concentration against relaxation activity of DNA topoisomerase I by detecting the conversion of supercoiled pBR322 DNA to its relaxed formMore data for this Ligand-Target Pair
Affinity DataIC50: 1.69E+4nMAssay Description:Inhibitory concentration against relaxation activity of DNA topoisomerase I by detecting the conversion of supercoiled pBR322 DNA to its relaxed formMore data for this Ligand-Target Pair
Affinity DataIC50: 3.44E+4nMAssay Description:Inhibitory concentration against relaxation activity of DNA topoisomerase I by detecting the conversion of supercoiled pBR322 DNA to its relaxed formMore data for this Ligand-Target Pair
Affinity DataIC50: 4.32E+4nMAssay Description:Inhibitory concentration against relaxation activity of DNA topoisomerase I by detecting the conversion of supercoiled pBR322 DNA to its relaxed formMore data for this Ligand-Target Pair
Affinity DataIC50: 8.80E+4nMAssay Description:Inhibitory concentration against relaxation activity of DNA topoisomerase I by detecting the conversion of supercoiled pBR322 DNA to its relaxed formMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibitory concentration against relaxation activity of DNA topoisomerase I by detecting the conversion of supercoiled pBR322 DNA to its relaxed formMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibitory concentration against relaxation activity of DNA topoisomerase I by detecting the conversion of supercoiled pBR322 DNA to its relaxed formMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibitory concentration against relaxation activity of DNA topoisomerase I by detecting the conversion of supercoiled pBR322 DNA to its relaxed formMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibitory concentration against relaxation activity of DNA topoisomerase I by detecting the conversion of supercoiled pBR322 DNA to its relaxed formMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibitory concentration against relaxation activity of DNA topoisomerase I by detecting the conversion of supercoiled pBR322 DNA to its relaxed formMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibitory concentration against relaxation activity of DNA topoisomerase I by detecting the conversion of supercoiled pBR322 DNA to its relaxed formMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibitory concentration against relaxation activity of DNA topoisomerase I by detecting the conversion of supercoiled pBR322 DNA to its relaxed formMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibitory concentration against relaxation activity of DNA topoisomerase I by detecting the conversion of supercoiled pBR322 DNA to its relaxed formMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibitory concentration against relaxation activity of DNA topoisomerase I by detecting the conversion of supercoiled pBR322 DNA to its relaxed formMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibitory concentration against relaxation activity of DNA topoisomerase I by detecting the conversion of supercoiled pBR322 DNA to its relaxed formMore data for this Ligand-Target Pair