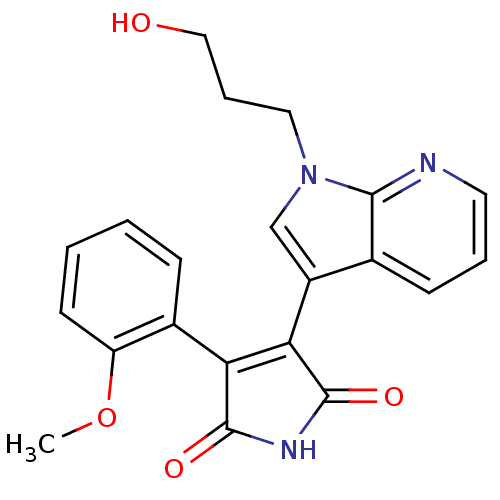
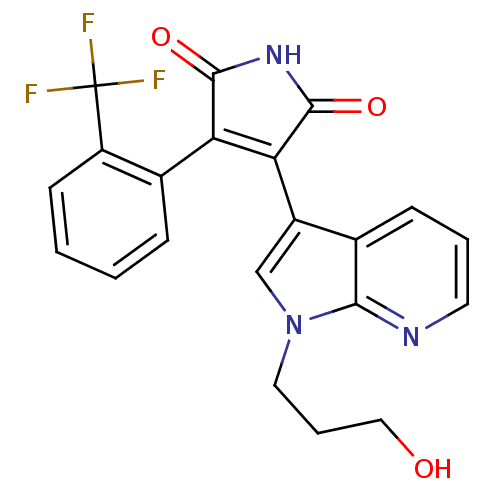
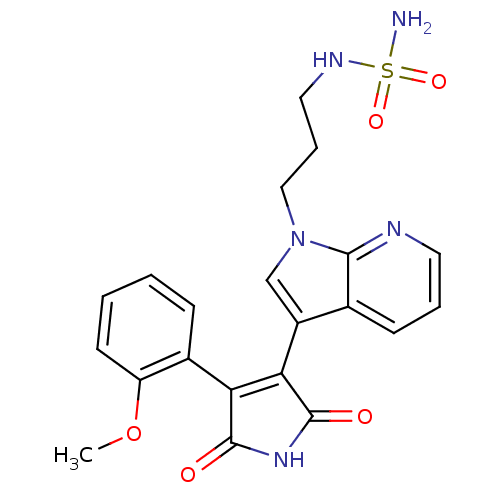
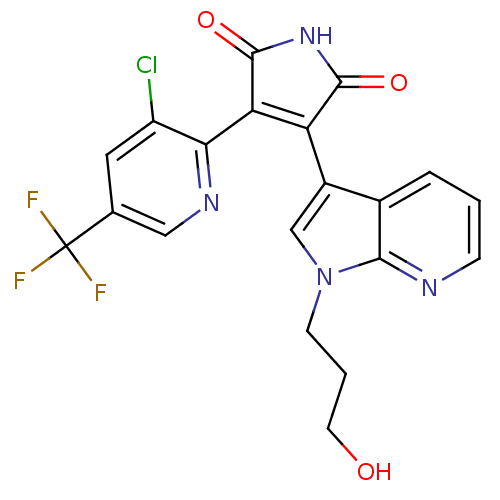
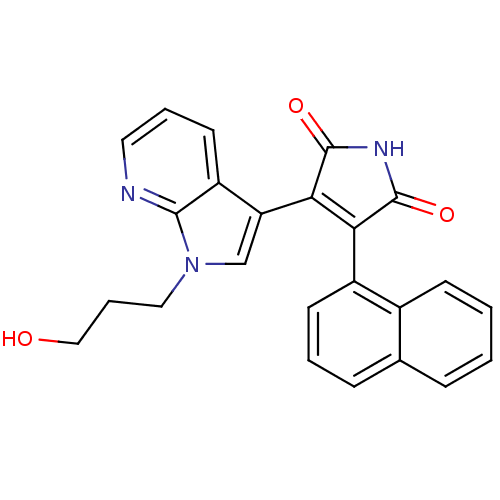
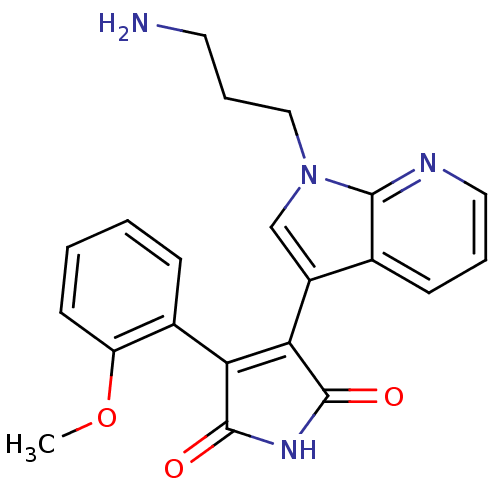
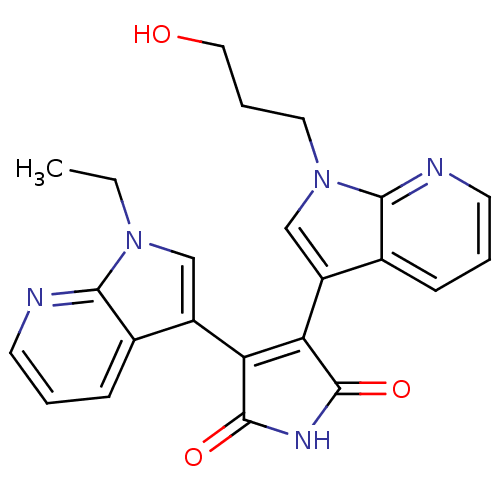
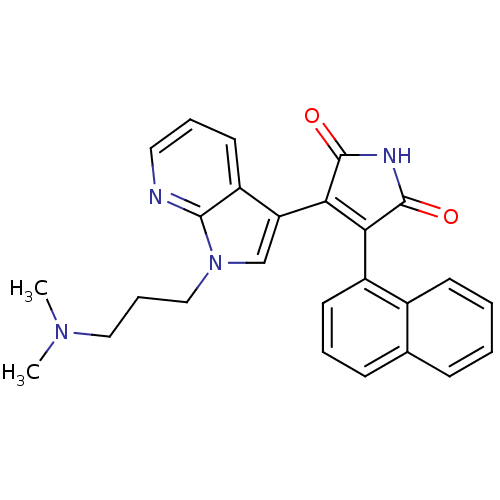
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibitory concentration against rabbit glycogen synthase kinase-3 beta using protein phosphatase inhibitor-2 as substrateMore data for this Ligand-Target Pair
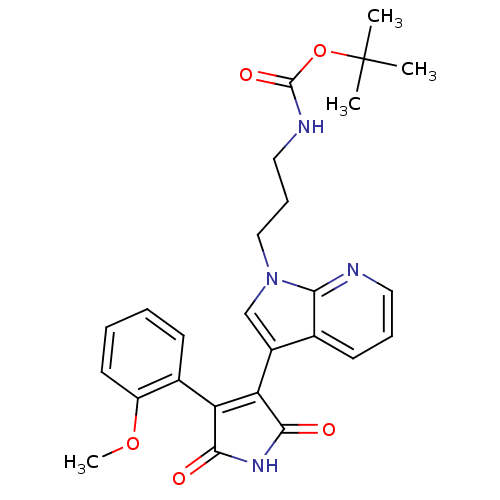
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibitory concentration against rabbit glycogen synthase kinase-3 beta using protein phosphatase inhibitor-2 as substrateMore data for this Ligand-Target Pair
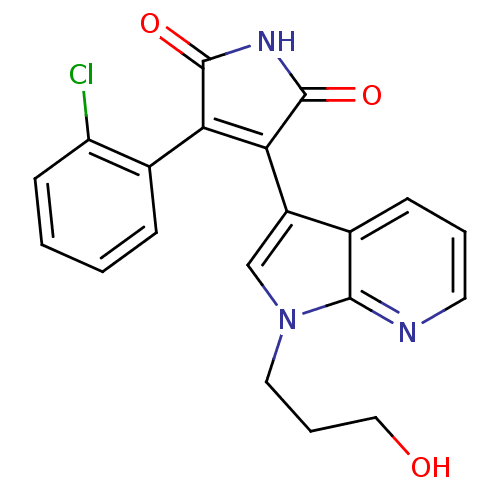
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 7nMAssay Description:Inhibitory concentration against rabbit glycogen synthase kinase-3 beta using protein phosphatase inhibitor-2 as substrateMore data for this Ligand-Target Pair
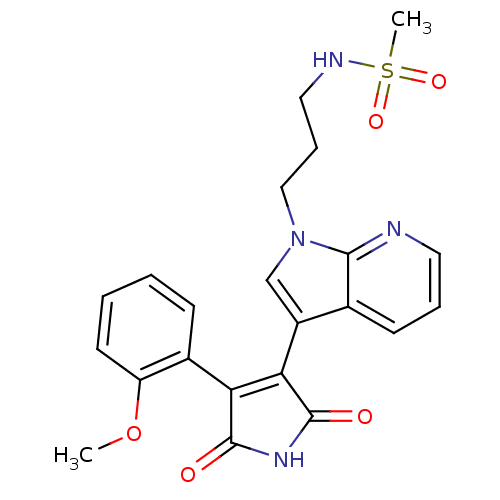
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 8nMAssay Description:Inhibitory concentration against rabbit glycogen synthase kinase-3 beta using protein phosphatase inhibitor-2 as substrateMore data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Inhibitory concentration against rabbit glycogen synthase kinase-3 beta using protein phosphatase inhibitor-2 as substrateMore data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 14nMAssay Description:Inhibitory concentration against rabbit glycogen synthase kinase-3 beta using protein phosphatase inhibitor-2 as substrateMore data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 14nMAssay Description:Inhibitory concentration against rabbit glycogen synthase kinase-3 beta using protein phosphatase inhibitor-2 as substrateMore data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 15nMAssay Description:Inhibitory concentration against rabbit glycogen synthase kinase-3 beta using protein phosphatase inhibitor-2 as substrateMore data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 20nMAssay Description:Inhibitory concentration against rabbit glycogen synthase kinase-3 beta using protein phosphatase inhibitor-2 as substrateMore data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 26nMAssay Description:Inhibitory concentration against rabbit glycogen synthase kinase-3 beta using protein phosphatase inhibitor-2 as substrateMore data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 31nMAssay Description:Inhibitory concentration against rabbit glycogen synthase kinase-3 beta using protein phosphatase inhibitor-2 as substrateMore data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 31nMAssay Description:Inhibitory concentration against rabbit glycogen synthase kinase-3 beta using protein phosphatase inhibitor-2 as substrateMore data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 37nMAssay Description:Inhibitory concentration against rabbit glycogen synthase kinase-3 beta using protein phosphatase inhibitor-2 as substrateMore data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 45nMAssay Description:Inhibitory concentration against rabbit glycogen synthase kinase-3 beta using protein phosphatase inhibitor-2 as substrateMore data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 65nMAssay Description:Inhibitory concentration against rabbit glycogen synthase kinase-3 beta using protein phosphatase inhibitor-2 as substrateMore data for this Ligand-Target Pair
TargetCyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 68nMAssay Description:Inhibition of Cyclin-dependent kinase 2-cyclin AMore data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 140nMAssay Description:Inhibitory concentration against rabbit glycogen synthase kinase-3 beta using protein phosphatase inhibitor-2 as substrateMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 1/G2/mitotic-specific cyclin-B1/G2/mitotic-specific cyclin-B2/G2/mitotic-specific cyclin-B3(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 170nMAssay Description:Inhibition of Cyclin-dependent kinase 1-cyclin BMore data for this Ligand-Target Pair
TargetProtein kinase C beta type(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 240nMAssay Description:Inhibitory concentration against human protein kinase C-betaII using histone as substrateMore data for this Ligand-Target Pair
TargetProtein kinase C beta type(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 270nMAssay Description:Inhibitory concentration against human protein kinase C-betaII using histone as substrateMore data for this Ligand-Target Pair
TargetCyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 430nMAssay Description:Inhibition of Cyclin-dependent kinase 2-cyclin AMore data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 480nMAssay Description:Inhibitory concentration against rabbit glycogen synthase kinase-3 beta using protein phosphatase inhibitor-2 as substrateMore data for this Ligand-Target Pair
Target3-phosphoinositide-dependent protein kinase 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
TargetRibosomal protein S6 kinase alpha-3(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 640nMAssay Description:Inhibition of Ribosomal S6 kinase 3More data for this Ligand-Target Pair
TargetCyclin-dependent kinase 1/G2/mitotic-specific cyclin-B1/G2/mitotic-specific cyclin-B2/G2/mitotic-specific cyclin-B3(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 700nMAssay Description:Inhibition of Cyclin-dependent kinase 1-cyclin BMore data for this Ligand-Target Pair
TargetProtein kinase C beta type(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 730nMAssay Description:Inhibitory concentration against human protein kinase C-betaII using histone as substrateMore data for this Ligand-Target Pair
TargetProtein kinase C beta type(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 890nMAssay Description:Inhibitory concentration against human protein kinase C-betaII using histone as substrateMore data for this Ligand-Target Pair
TargetProtein kinase C beta type(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 1.07E+3nMAssay Description:Inhibitory concentration against human protein kinase C-betaII using histone as substrateMore data for this Ligand-Target Pair
TargetProtein kinase C beta type(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibitory concentration against human protein kinase C-betaII using histone as substrateMore data for this Ligand-Target Pair
TargetProtein kinase C beta type(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 1.16E+3nMAssay Description:Inhibitory concentration against human protein kinase C-betaII using histone as substrateMore data for this Ligand-Target Pair
TargetProtein kinase C alpha type(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 1.40E+3nMAssay Description:Inhibitory concentration against protein kinase C-alpha using histone as substrateMore data for this Ligand-Target Pair
TargetProtein kinase C beta type(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 1.44E+3nMAssay Description:Inhibitory concentration against human protein kinase C-betaII using histone as substrateMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lyn(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 1.80E+3nMAssay Description:Inhibition of Protein tyrosine kinase LynMore data for this Ligand-Target Pair
TargetProtein kinase C beta type(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 1.85E+3nMAssay Description:Inhibitory concentration against human protein kinase C-betaII using histone as substrateMore data for this Ligand-Target Pair
TargetProtein kinase C beta type(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 1.88E+3nMAssay Description:Inhibitory concentration against human protein kinase C-betaII using histone as substrateMore data for this Ligand-Target Pair
TargetRibosomal protein S6 kinase alpha-3(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of Ribosomal S6 kinase 3More data for this Ligand-Target Pair
TargetProtein kinase C beta type(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 2.26E+3nMAssay Description:Inhibitory concentration against human protein kinase C-betaII using histone as substrateMore data for this Ligand-Target Pair
TargetProtein kinase C beta type(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 2.38E+3nMAssay Description:Inhibitory concentration against human protein kinase C-betaII using histone as substrateMore data for this Ligand-Target Pair
TargetProtein kinase C beta type(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 2.73E+3nMAssay Description:Inhibitory concentration against human protein kinase C-betaII using histone as substrateMore data for this Ligand-Target Pair
TargetProtein kinase C gamma type(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 2.80E+3nMAssay Description:Inhibition of protein kinase C-gammaMore data for this Ligand-Target Pair
TargetCyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 2.80E+3nMAssay Description:Inhibition of Cyclin-dependent kinase 2-cyclin AMore data for this Ligand-Target Pair
TargetProtein kinase C gamma type(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 3.60E+3nMAssay Description:Inhibition of protein kinase C-gammaMore data for this Ligand-Target Pair
TargetProtein kinase C alpha type(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 3.60E+3nMAssay Description:Inhibitory concentration against protein kinase C-alpha using histone as substrateMore data for this Ligand-Target Pair
TargetProtein kinase C alpha type(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 4.00E+3nMAssay Description:Inhibitory concentration against protein kinase C-alpha using histone as substrateMore data for this Ligand-Target Pair
TargetProtein kinase C beta type(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 4.36E+3nMAssay Description:Inhibitory concentration against human protein kinase C-betaII using histone as substrateMore data for this Ligand-Target Pair
TargetProtein kinase C alpha type(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 4.40E+3nMAssay Description:Inhibitory concentration against protein kinase C-alpha using histone as substrateMore data for this Ligand-Target Pair
TargetProtein kinase C alpha type(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 4.50E+3nMAssay Description:Inhibitory concentration against protein kinase C-alpha using histone as substrateMore data for this Ligand-Target Pair
TargetProtein kinase C alpha type(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 4.70E+3nMAssay Description:Inhibitory concentration against protein kinase C-alpha using histone as substrateMore data for this Ligand-Target Pair
TargetProtein kinase C gamma type(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 5.50E+3nMAssay Description:Inhibition of protein kinase C-gammaMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 1/G2/mitotic-specific cyclin-B1/G2/mitotic-specific cyclin-B2/G2/mitotic-specific cyclin-B3(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 5.80E+3nMAssay Description:Inhibition of Cyclin-dependent kinase 1-cyclin BMore data for this Ligand-Target Pair