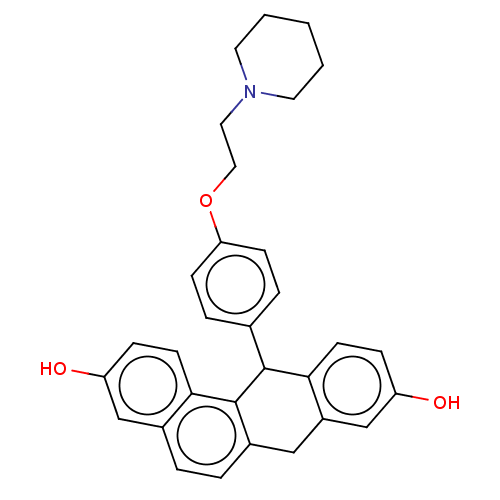
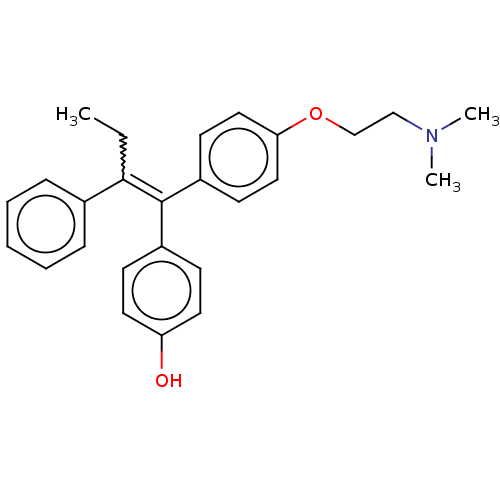
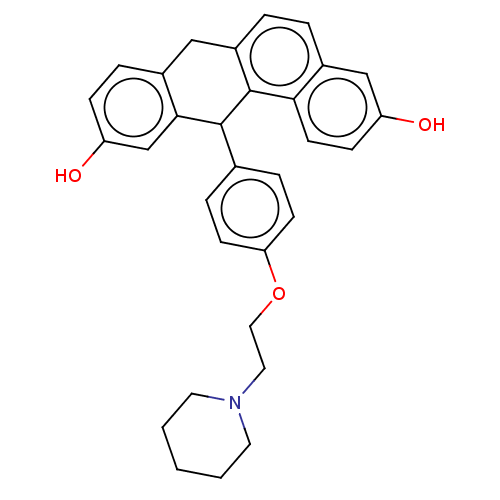
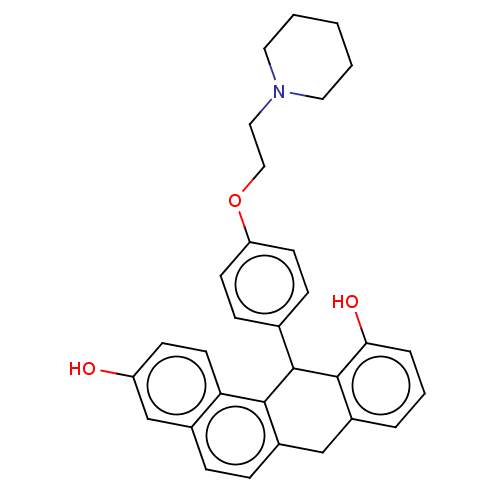
Affinity DataIC50: 0.700nMAssay Description:Antagonist effect against 10 pM 17-beta-estradiol induced MCF-7 cell proliferationMore data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:Antagonist effect against 10 pM 17-beta-estradiol induced MCF-7 cell proliferationMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Antagonist effect against 10 pM 17-beta-estradiol induced MCF-7 cell proliferationMore data for this Ligand-Target Pair
Affinity DataIC50: 2.40nMAssay Description:Antagonist effect against 10 pM 17-beta-estradiol induced MCF-7 cell proliferationMore data for this Ligand-Target Pair
Affinity DataIC50: 2.60nMAssay Description:Antagonist effect against 10 pM 17-beta-estradiol induced MCF-7 cell proliferationMore data for this Ligand-Target Pair
Affinity DataIC50: 2.90nMAssay Description:Antagonist effect against 10 pM 17-beta-estradiol induced MCF-7 cell proliferationMore data for this Ligand-Target Pair
Affinity DataIC50: 6.60nMAssay Description:Antagonist effect against 10 pM 17-beta-estradiol induced MCF-7 cell proliferationMore data for this Ligand-Target Pair
Affinity DataIC50: 17nMAssay Description:Antagonist effect against 10 pM 17-beta-estradiol induced MCF-7 cell proliferationMore data for this Ligand-Target Pair
Affinity DataIC50: 23nMAssay Description:Antagonist effect against 10 pM 17-beta-estradiol induced MCF-7 cell proliferationMore data for this Ligand-Target Pair
Affinity DataIC50: 23nMAssay Description:Antagonist effect against 10 pM 17-beta-estradiol induced MCF-7 cell proliferationMore data for this Ligand-Target Pair
Affinity DataIC50: 28nMAssay Description:Antagonist activity as inhibition of 1 nM 17-beta-estradiol stimulated alkaline phosphatase induction in Ishikawa endometrial cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 42nMAssay Description:Antagonist effect against 10 pM 17-beta-estradiol induced MCF-7 cell proliferationMore data for this Ligand-Target Pair
Affinity DataIC50: 45nMAssay Description:Antagonist activity as inhibition of 1 nM 17-beta-estradiol stimulated alkaline phosphatase induction in Ishikawa endometrial cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:Antagonist activity as inhibition of 1 nM 17-beta-estradiol stimulated alkaline phosphatase induction in Ishikawa endometrial cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 53nMAssay Description:Antagonist activity as inhibition of 1 nM 17-beta-estradiol stimulated alkaline phosphatase induction in Ishikawa endometrial cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 57nMAssay Description:Antagonist effect against 10 pM 17-beta-estradiol induced MCF-7 cell proliferationMore data for this Ligand-Target Pair
Affinity DataIC50: 68nMAssay Description:Antagonist effect against 10 pM 17-beta-estradiol induced MCF-7 cell proliferationMore data for this Ligand-Target Pair
Affinity DataIC50: 130nMAssay Description:Antagonist activity as inhibition of 1 nM 17-beta-estradiol stimulated alkaline phosphatase induction in Ishikawa endometrial cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 160nMAssay Description:Antagonist activity as inhibition of 1 nM 17-beta-estradiol stimulated alkaline phosphatase induction in Ishikawa endometrial cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 160nMAssay Description:Antagonist activity as inhibition of 1 nM 17-beta-estradiol stimulated alkaline phosphatase induction in Ishikawa endometrial cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 510nMAssay Description:Antagonist activity as inhibition of 1 nM 17-beta-estradiol stimulated alkaline phosphatase induction in Ishikawa endometrial cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 580nMAssay Description:Antagonist activity as inhibition of 1 nM 17-beta-estradiol stimulated alkaline phosphatase induction in Ishikawa endometrial cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 680nMAssay Description:Antagonist activity as inhibition of 1 nM 17-beta-estradiol stimulated alkaline phosphatase induction in Ishikawa endometrial cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 690nMAssay Description:Antagonist activity as inhibition of 1 nM 17-beta-estradiol stimulated alkaline phosphatase induction in Ishikawa endometrial cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 710nMAssay Description:Antagonist activity as inhibition of 1 nM 17-beta-estradiol stimulated alkaline phosphatase induction in Ishikawa endometrial cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 820nMAssay Description:Antagonist activity as inhibition of 1 nM 17-beta-estradiol stimulated alkaline phosphatase induction in Ishikawa endometrial cellsMore data for this Ligand-Target Pair
Affinity DataEC50: 3.5nMAssay Description:Agonist activity as alkaline phosphatase induction in Ishikawa endometrial cells compared to E2More data for this Ligand-Target Pair
Affinity DataEC50: 10nMAssay Description:Agonist activity as alkaline phosphatase induction in Ishikawa endometrial cells compared to E2More data for this Ligand-Target Pair
Affinity DataEC50: 3.10nMAssay Description:Agonist activity as alkaline phosphatase induction in Ishikawa endometrial cells compared to E2More data for this Ligand-Target Pair
Affinity DataEC50: 0.900nMAssay Description:Agonist activity as alkaline phosphatase induction in Ishikawa endometrial cells compared to E2More data for this Ligand-Target Pair
Affinity DataEC50: 0.800nMAssay Description:Agonist activity as alkaline phosphatase induction in Ishikawa endometrial cells compared to E2More data for this Ligand-Target Pair
Affinity DataEC50: 1.60nMAssay Description:Agonist activity as alkaline phosphatase induction in Ishikawa endometrial cells compared to E2More data for this Ligand-Target Pair
Affinity DataEC50: 3.10nMAssay Description:Agonist activity as alkaline phosphatase induction in Ishikawa endometrial cells compared to E2More data for this Ligand-Target Pair
Affinity DataEC50: 0.5nMAssay Description:Agonist activity as alkaline phosphatase induction in Ishikawa endometrial cells compared to E2More data for this Ligand-Target Pair
Affinity DataEC50: 0.700nMAssay Description:Agonist activity as alkaline phosphatase induction in Ishikawa endometrial cells compared to E2More data for this Ligand-Target Pair
Affinity DataEC50: 3.10nMAssay Description:Agonist activity as alkaline phosphatase induction in Ishikawa endometrial cells compared to E2More data for this Ligand-Target Pair
Affinity DataEC50: 0.400nMAssay Description:Agonist activity as alkaline phosphatase induction in Ishikawa endometrial cells compared to E2More data for this Ligand-Target Pair
Affinity DataEC50: 28nMAssay Description:Agonist activity as alkaline phosphatase induction in Ishikawa endometrial cells compared to E2More data for this Ligand-Target Pair
Affinity DataEC50: 2.60nMAssay Description:Agonist activity as alkaline phosphatase induction in Ishikawa endometrial cells compared to E2More data for this Ligand-Target Pair