Reaction Details  Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataCell Reactant:
Transcriptional Regulator TtgR
Syringe Reactant:
BDBM7458
Meas. Tech.:
Isothermal Titration Calorimetry
Entry Date.:
07/23/08
ΔG°:
-27.8806±0.209 (kJ/mole)
pH:
7±n/a
Log10Kb:
3.7
Temperature:
303.15±n/a (K)
ΔHobs :
-5.3504±0.1672 (kJ/mole)
Corrected for ΔHioniz:
no
ΔS° :
0.0743±0.0008 (kJ/mole-K)
Citation
Cell React
Source:
A 651-bp fragment containing the ttgR gene was amplified by PCR from P. putida DOT-T1E chromosomal DNA, and cloned in E. coli expression vector. TtgR protein was over-expressed and purified.
Prep. Method:
TtgR protein was further purified by size exclusion chromatography using a Sephacryl HR-200 column (Amersham Biosciences). Eluted fractions of TtgR were pooled, concentrated, and dialyzed against the buffer.
Name:
Transcriptional Regulator TtgR
Synonyms:
HTH-type transcriptional regulator ttgR | TTGR_PSEPT | Toluene efflux pump ttgABC operon repressor | ttgR
Type:
Repressor; homodimer
Mol. Mass.:
23852.57
Organism:
Pseudomonas putida
Description:
n/a
Residue:
210
Sequence:
MVRRTKEEAQETRAQIIEAAERAFYKRGVARTTLADIAELAGVTRGAIYWHFNNKAELVQALLDSLHETHDHLARASESEDEVDPLGCMRKLLLQVFNELVLDARTRRINEILHHKCEFTDDMCEIRQQRQSAVLDCHKGITLALANAVRRGQLPGELDAERAAVAMFAYVDGLIRRWLLLPDSVDLLGDVEKWVDTGLDMLRLSPALRK
Syringe React
Prep. Method:
All chemicals were manipulated in glass vessels and effector samples were neither degassed nor filtered, to avoid evaporation or nonspecific binding. Analyses were carried out using 5% DMSO to facilitate solubilization.
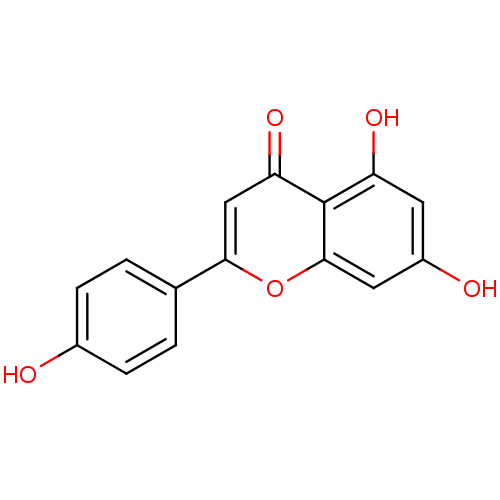
Name:
BDBM7458
Synonyms:
5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one | 5,7-dihydroxy-2-(4-hydroxyphenyl)-chromen-4-one | Apigenin | Apigenin (2) | Apigenin (3) | Apigenin (7) | Apigenin, 13 | CHEMBL28 | Naringenin, 18 | US10278929, Apigenin | US11337935, Compound Apigenin | acs.jmedchem.1c00409_ST.789 | cid_5280443 | jm5b01461, Compound 90
Type:
Small organic molecule
Emp. Form.:
C15H10O5
Mol. Mass.:
270.2369
SMILES:
Oc1ccc(cc1)-c1cc(=O)c2c(O)cc(O)cc2o1