Reaction Details  Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataCell Reactant:
3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMG-CoA)
Syringe Reactant:
BDBM24423
Meas. Tech.:
Isothermal Titration Calorimetry
Entry Date.:
09/17/08
ΔG°:
-43.054±n/a (kJ/mole)
pH:
7.2±n/a
Temperature:
303.15±n/a (K)
ΔHobs :
-31.768±n/a (kJ/mole)
Corrected for ΔHioniz:
no
ΔS° :
0.0372±n/a (kJ/mole-K)
Citation
 Cywin, CL; Sarver, RW; Klunder, JM; Bills, E; Hoermann, M; Bolton, G; Bratton, LD; Brickwood, JR; Caspers, NL; David, E; Dunbar, JB; Grob, PM; Schwartz, R; Harris, MS; Pauletti, D; Hutchings, RH; Barringer, KJ; Kennedy, RM; Shih, CK; Larsen, SD; Pavlovsky, A; Sorge, CL; Erickson, DA; Pfefferkorn, JA; Joseph, DP; Bainbridge, G; Hattox, SE Thermodynamic and structure guided design of statin based inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A reductase. J Med Chem 51:3804-13 (2008) [PubMed] Article
Cywin, CL; Sarver, RW; Klunder, JM; Bills, E; Hoermann, M; Bolton, G; Bratton, LD; Brickwood, JR; Caspers, NL; David, E; Dunbar, JB; Grob, PM; Schwartz, R; Harris, MS; Pauletti, D; Hutchings, RH; Barringer, KJ; Kennedy, RM; Shih, CK; Larsen, SD; Pavlovsky, A; Sorge, CL; Erickson, DA; Pfefferkorn, JA; Joseph, DP; Bainbridge, G; Hattox, SE Thermodynamic and structure guided design of statin based inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A reductase. J Med Chem 51:3804-13 (2008) [PubMed] ArticleCell React
Source:
HMGR was expressed as an N-terminal His6 protein truncated from amino acid 441-875 with the mutation M485I in the Escherichia coli.
Prep. Method:
Protein was purified via Ni-affinity and gel filtration chromatography.
Name:
3-hydroxy-3-methylglutaryl-coenzyme A reductase
Synonyms:
3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase | 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMG-CoA) | HMDH_HUMAN | HMG-CoA Reductase | HMG-CoA reductase (HMGR) | HMGCR
Type:
Enzyme
Mol. Mass.:
97477.10
Organism:
Homo sapiens (Human)
Description:
P04035
Residue:
888
Sequence:
MLSRLFRMHGLFVASHPWEVIVGTVTLTICMMSMNMFTGNNKICGWNYECPKFEEDVLSSDIIILTITRCIAILYIYFQFQNLRQLGSKYILGIAGLFTIFSSFVFSTVVIHFLDKELTGLNEALPFFLLLIDLSRASTLAKFALSSNSQDEVRENIARGMAILGPTFTLDALVECLVIGVGTMSGVRQLEIMCCFGCMSVLANYFVFMTFFPACVSLVLELSRESREGRPIWQLSHFARVLEEEENKPNPVTQRVKMIMSLGLVLVHAHSRWIADPSPQNSTADTSKVSLGLDENVSKRIEPSVSLWQFYLSKMISMDIEQVITLSLALLLAVKYIFFEQTETESTLSLKNPITSPVVTQKKVPDNCCRREPMLVRNNQKCDSVEEETGINRERKVEVIKPLVAETDTPNRATFVVGNSSLLDTSSVLVTQEPEIELPREPRPNEECLQILGNAEKGAKFLSDAEIIQLVNAKHIPAYKLETLMETHERGVSIRRQLLSKKLSEPSSLQYLPYRDYNYSLVMGACCENVIGYMPIPVGVAGPLCLDEKEFQVPMATTEGCLVASTNRGCRAIGLGGGASSRVLADGMTRGPVVRLPRACDSAEVKAWLETSEGFAVIKEAFDSTSRFARLQKLHTSIAGRNLYIRFQSRSGDAMGMNMISKGTEKALSKLHEYFPEMQILAVSGNYCTDKKPAAINWIEGRGKSVVCEAVIPAKVVREVLKTTTEAMIEVNINKNLVGSAMAGSIGGYNAHAANIVTAIYIACGQDAAQNVGSSNCITLMEASGPTNEDLYISCTMPSIEIGTVGGGTNLLPQQACLQMLGVQGACKDNPGENARQLARIVCGTVMAGELSLMAALAAGHLVKSHMIHNRSKINLQDLQGACTKKTA
Syringe React
Prep. Method:
To minimize heat of dilution effects resulting from differences in buffer composition between ligand and protein, ligands were dissolved in dialysate buffer from the final step in the HMGR purification.
Name:
BDBM24423
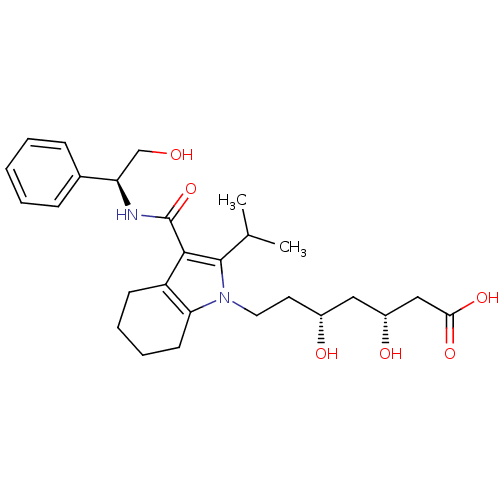
Synonyms:
(3R,5R)-3,5-dihydroxy-7-(3-{[(1S)-2-hydroxy-1-phenylethyl]carbamoyl}-2-(propan-2-yl)-4,5,6,7-tetrahydro-1H-indol-1-yl)heptanoic acid | Bicyclic Pyrrole Series 3 Compound, 10
Type:
Small organic molecule
Emp. Form.:
C27H38N2O6
Mol. Mass.:
486.6004
SMILES:
CC(C)c1c(C(=O)N[C@H](CO)c2ccccc2)c2CCCCc2n1CC[C@@H](O)C[C@@H](O)CC(O)=O |r|