Reaction Details  Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataCell Reactant:
DNA Gyrase
Syringe Reactant:
BDBM284
Meas. Tech.:
Isothermal Titration Calorimetry
Entry Date.:
03/31/03
ΔG°:
-40.3±0.5 (kJ/mole)
pH:
7.5±n/a
Log10Kb:
6.3
Temperature:
298.15±n/a (K)
ΔH° :
-18.9±3.1 (kJ/mole)
ΔHobs :
-18.9±3.1 (kJ/mole)
Corrected for ΔHioniz:
not known
ΔCp :
-0.83±0.07 (kJ/mole)
ΔS° :
0.0718±0.0054 (kJ/mole-K)
Citation
 Lafitte, D; Lamour, V; Tsvetkov, PO; Makarov, AA; Klich, M; Deprez, P; Moras, D; Briand, C; Gilli, R DNA gyrase interaction with coumarin-based inhibitors: the role of the hydroxybenzoate isopentenyl moiety and the 5'-methyl group of the noviose. Biochemistry 41:7217-23 (2002) [PubMed] Article
Lafitte, D; Lamour, V; Tsvetkov, PO; Makarov, AA; Klich, M; Deprez, P; Moras, D; Briand, C; Gilli, R DNA gyrase interaction with coumarin-based inhibitors: the role of the hydroxybenzoate isopentenyl moiety and the 5'-methyl group of the noviose. Biochemistry 41:7217-23 (2002) [PubMed] ArticleCell React
Source:
Bacterial clone
Purity:
97%
Prep. Method:
Gilbert, E. J., and Maxwell, A. (1994) Mol. Microbiol. 12, 365-373.
Name:
DNA gyrase subunit B
Synonyms:
gyrB
Type:
Enzyme
Mol. Mass.:
89941.28
Organism:
Escherichia coli
Description:
C3SLN3
Residue:
804
Sequence:
MSNSYDSSSIKVLKGLDAVRKRPGMYIGDTDDGTGLHHMVFEVVDNAIDEALAGHCKEIIVTIHADNSVSVQDDGRGIPTGIHPEEGVSAAEVIMTVLHAGGKFDDNSYKVSGGLHGVGVSVVNALSQKLELVIQREGKIHRQIYEHGVPQAPLAVTGETEKTGTMVRFWPSLETFTNVTEFEYEILAKRLRELSFLNSGVSIRLRDKRDGKEDHFHYEGGIKAFVEYLNKNKTPIHPNIFYFSTEKDGIGVEVALQWNDGFQENIYCFTNNIPQRDGGTHLAGFRAAMTRTLNAYMDKEGYSKKAKVSATGDDAREGLIAVVSVKVPDPKFSSQTKDKLVSSEVKSAVEQQMNELLAEYLLENPTDAKIVVGKIIDAARAREAARRAREMTRRKGALDLAGLPGKLADCQERDPALSELYLVEGDSAGGSAKQGRNRKNQAILPLKGKILNVEKARFDKMLSSQEVATLITALGCGIGRDEYNPDKLRYHSIIIMTDADVDGSHIRTLLLTFFYRQMPEIVERGHVYIAQPPLYKVKKGKQEQYIKDDEAMDQYQISIALDGATLHTNASAPALAGEALEKLVSEYNATQKMINRMERRYPKAMLKELIYQPTLTEADLSDEQTVTRWVNALVSELNDKEQHGSQWKFDVHTNAEQNLFEPIVRVRTHGVDTDYPLDHEFITGGEYRRICTLGEKLRGLLEEDAFIERGERRQPVASFEQALDWLVKESRRGLSIQRYKGLGEMNPEQLWETTMDPESRRMLRVTVKDAIAADQLFTTLMGDAVEPRRAFIEENALKAANIDI
Syringe React
Source:
Synthesis
Prep. Method:
Ferroud, D., Collard, J., Klich, M., Dupuis-Hamelin, C., Mauvais, P., Lassaigne, P., Bonnefoy, A., and Musicki, B.(1999) Bioorg. Med. Chem. Lett. 9, 2881-2886.
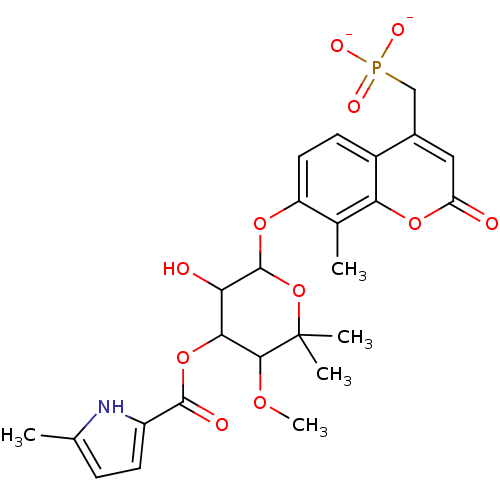
Name:
BDBM284
Synonyms:
Coumarin-Based, DNA Gyrase Inhibitor | RU 64135 | {[7-({3-hydroxy-5-methoxy-6,6-dimethyl-4-[(5-methyl-1H-pyrrol-2-yl)carbonyloxy]oxan-2-yl}oxy)-8-methyl-2-oxo-2H-chromen-4-yl]methyl}phosphonate
Type:
Antibiotic
Emp. Form.:
C25H28NO11P
Mol. Mass.:
549.4648
SMILES:
COC1C(OC(=O)c2ccc(C)[nH]2)C(O)C(Oc2ccc3c(CP([O-])([O-])=O)cc(=O)oc3c2C)OC1(C)C