Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Hexokinase HKDC1 [W721R]
Ligand
BDBM80083
Substrate
n/a
Meas. Tech.
Dose Response confirmation of activators of hexokinase domain containing I (HKDC1)
EC50
33100±n/a nM
Citation
 PubChem, PC Dose Response confirmation of activators of hexokinase domain containing I (HKDC1) PubChem Bioassay (2011)[AID]
PubChem, PC Dose Response confirmation of activators of hexokinase domain containing I (HKDC1) PubChem Bioassay (2011)[AID] More Info.:
Target
Name:
Hexokinase HKDC1 [W721R]
Synonyms:
HKDC1 | HKDC1_HUMAN | putative hexokinase HKDC1
Type:
Enzyme Catalytic Domain
Mol. Mass.:
102520.24
Organism:
Homo sapiens (Human)
Description:
gi_156151420
Residue:
917
Sequence:
MFAVHLMAFYFSKLKEDQIKKVDRFLYHMRLSDDTLLDIMRRFRAEMEKGLAKDTNPTAAVKMLPTFVRAIPDGSENGEFLSLDLGGSKFRVLKVQVAEEGKRHVQMESQFYPTPNEIIRGNGTELFEYVADCLADFMKTKDLKHKKLPLGLTFSFPCRQTKLEEGVLLSWTKKFKARGVQDTDVVSRLTKAMRRHKDMDVDILALVNDTVGTMMTCAYDDPYCEVGVIIGTGTNACYMEDMSNIDLVEGDEGRMCINTEWGAFGDDGALEDIRTEFDRELDLGSLNPGKQLFEKMISGLYLGELVRLILLKMAKAGLLFGGEKSSALHTKGKIETRHVAAMEKYKEGLANTREILVDLGLEPSEADCIAVQHVCTIVSFRSANLCAAALAAILTRLRENKKVERLRTTVGMDGTLYKIHPQYPKRLHKVVRKLVPSCDVRFLLSESGSTKGAAMVTAVASRVQAQRKQIDRVLALFQLTREQLVDVQAKMRAELEYGLKKKSHGLATVRMLPTYVCGLPDGTEKGKFLALDLGGTNFRVLLVKIRSGRRSVRMYNKIFAIPLEIMQGTGEELFDHIVQCIADFLDYMGLKGASLPLGFTFSFPCRQMSIDKGTLIGWTKGFKATDCEGEDVVDMLREAIKRRNEFDLDIVAVVNDTVGTMMTCGYEDPNCEIGLIAGTGSNMCYMEDMRNIEMVEGGEGKMCINTEWGGFGDNGCIDDIRTRYDTEVDEGSLNPGKQRYEKMTSGMYLGEIVRQILIDLTKQGLLFRGQISERLRTRGIFETKFLSQIESDRLALLQVRRILQQLGLDSTCEDSIVVKEVCGAVSRRAAQLCGAGLAAIVEKRREDQGLEHLRITVGVDGTLYKLHPHFSRILQETVKELAPRCDVTFMLSEDGSGKGAALITAVAKRLQQAQKEN
Inhibitor
Name:
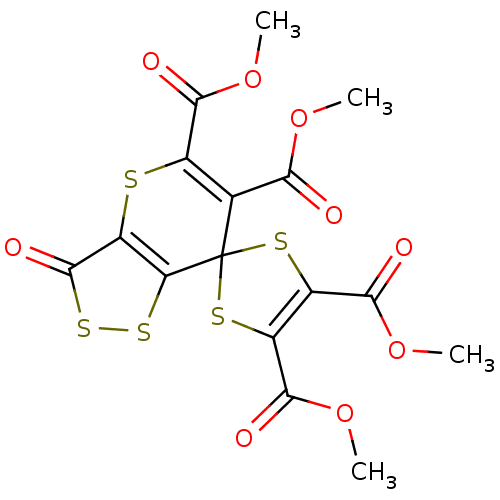
BDBM80083
Synonyms:
3'-ketospiro[1,3-dithiole-2,7'-dithiolo[4,3-b]thiopyran]-4,5,5',6'-tetracarboxylic acid tetramethyl ester | 3'-oxospiro[1,3-dithiole-2,7'-dithiolo[4,3-b]thiopyran]-4,5,5',6'-tetracarboxylic acid tetramethyl ester | MLS002702363 | SMR001565925 | cid_389753 | tetramethyl 3'-oxidanylidenespiro[1,3-dithiole-2,7'-[1,2]dithiolo[4,3-b]thiopyran]-4,5,5',6'-tetracarboxylate | tetramethyl 3'-oxospiro[1,3-dithiole-2,7'-dithiolo[4,3-b]thiopyran]-4,5,5',6'-tetracarboxylate
Type:
Small organic molecule
Emp. Form.:
C16H12O9S5
Mol. Mass.:
508.586
SMILES:
COC(=O)C1=C(SC2(S1)c1ssc(=O)c1SC(C(=O)OC)=C2C(=O)OC)C(=O)OC |c:22,t:4|