Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Leukotriene A-4 hydrolase
Ligand
BDBM50272141
Substrate
n/a
Meas. Tech.
ChEMBL_510221 (CHEMBL1004837)
IC50
1100±n/a nM
Citation
 Enomoto, H; Morikawa, Y; Miyake, Y; Tsuji, F; Mizuchi, M; Suhara, H; Fujimura, K; Horiuchi, M; Ban, M Synthesis and biological evaluation of N-mercaptoacylproline and N-mercaptoacylthiazolidine-4-carboxylic acid derivatives as leukotriene A4 hydrolase inhibitors. Bioorg Med Chem Lett 18:4529-32 (2008) [PubMed] Article
Enomoto, H; Morikawa, Y; Miyake, Y; Tsuji, F; Mizuchi, M; Suhara, H; Fujimura, K; Horiuchi, M; Ban, M Synthesis and biological evaluation of N-mercaptoacylproline and N-mercaptoacylthiazolidine-4-carboxylic acid derivatives as leukotriene A4 hydrolase inhibitors. Bioorg Med Chem Lett 18:4529-32 (2008) [PubMed] Article More Info.:
Target
Name:
Leukotriene A-4 hydrolase
Synonyms:
LKHA4_CAVPO | LTA4H
Type:
PROTEIN
Mol. Mass.:
68972.78
Organism:
Cavia porcellus
Description:
ChEMBL_544515
Residue:
611
Sequence:
MPEVVDTCSLASPATVCRTKHLHLRCSVDFTRRALTGVAALTIQSQEDNLRSLILDTKDLTIEKVVINGQEVKYALGEKQSYKGSPMEISLPIALSKNQEVVIEISFETSPKSSALQWLTPEQTSGKEHPYLFSQCQAIHCRAFLPCQDTPSVKLTYTAEVSVPKELVALMSAIRDGEAPDPADPSRKIYKFSQKVPIPCYLIALVVGALESRKIGPRTLVWSEKEQVDKSAYEFSETESMLKIAEDLGGPYVWGQYDRLVLPPSFSYGGMENPCLTFVTPTLLAGDKSLSNVIAHEISHTWTGNLVTNKTWDHFWLNEGHTVYLERHICGRLFGEKFRHFHALGGWGELQNTVKTLGETQAFTKLVVDLTDTDPDVAYSSVPYEKGFALLFHLEQLLGGPEVFLGFLKAYVEKFSYKSITTDDWKNFLFSHFKDKVDILNQVDWDAWLYSPGLPPIKPNYDMTLTNACIALSQRWITAKEKDLNTFSATDLKDLSSHQVNEFLAQVLQRAPLPLGHVKRMQEVYNCNAINNSEIRFRWLRLCIQSKWEEAIPLALKMATEQGRMKFTRPLFKDLAAFDKSHDQAIQTYHAHKASMHPVTAMLVGKDLKVE
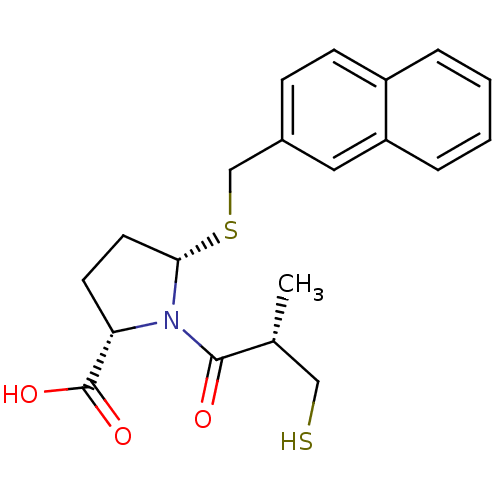
Inhibitor
Name:
BDBM50272141
Synonyms:
(2S,5S)-1-((S)-3-mercapto-2-methylpropanoyl)-5-(naphthalen-2-ylmethylthio)pyrrolidine-2-carboxylic acid | CHEMBL499610
Type:
Small organic molecule
Emp. Form.:
C20H23NO3S2
Mol. Mass.:
389.532
SMILES:
C[C@H](CS)C(=O)N1[C@H](CC[C@H]1C(O)=O)SCc1ccc2ccccc2c1 |r|