Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Cytochrome P450 3A4
Ligand
BDBM21278
Substrate
n/a
Meas. Tech.
ChEMBL_651866 (CHEMBL1227231)
IC50
>20±n/a nM
Citation
 Lee, J; Seo, HJ; Lee, SH; Kim, J; Jung, ME; Lee, SH; Song, KS; Lee, J; Kang, SY; Kim, MJ; Kim, MS; Son, EJ; Lee, M; Han, HK Discovery of 2-(4-((1H-1,2,4-triazol-1-yl)methyl)-5-(4-bromophenyl)-1-(2-chlorophenyl)-1H-pyrazol-3-yl)-5-tert-butyl-1,3,4-thiadiazole (GCC2680) as a potent, selective and orally efficacious cannabinoid-1 receptor antagonist. Bioorg Med Chem 18:6377-88 (2010) [PubMed] Article
Lee, J; Seo, HJ; Lee, SH; Kim, J; Jung, ME; Lee, SH; Song, KS; Lee, J; Kang, SY; Kim, MJ; Kim, MS; Son, EJ; Lee, M; Han, HK Discovery of 2-(4-((1H-1,2,4-triazol-1-yl)methyl)-5-(4-bromophenyl)-1-(2-chlorophenyl)-1H-pyrazol-3-yl)-5-tert-butyl-1,3,4-thiadiazole (GCC2680) as a potent, selective and orally efficacious cannabinoid-1 receptor antagonist. Bioorg Med Chem 18:6377-88 (2010) [PubMed] Article More Info.:
Target
Name:
Cytochrome P450 3A4
Synonyms:
Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase
Type:
Enzyme
Mol. Mass.:
57349.57
Organism:
Homo sapiens (Human)
Description:
n/a
Residue:
503
Sequence:
MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMFDMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISIAEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYSMDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICVFPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSIIFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVVNETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFSKKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLGGLLQPEKPVVLKVESRDGTVSGA
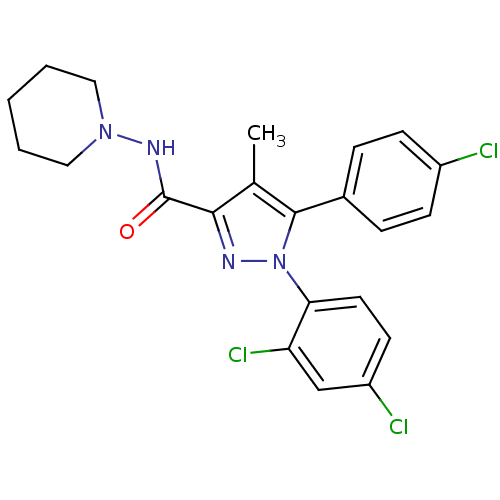
Inhibitor
Name:
BDBM21278
Synonyms:
5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide | Acomplia | CHEMBL111 | CHEMBL558598 | RIMONABANT HYDROCHLORIDE | Rimonabant | SR 141716A | SR141716 | SR141716A | [3H]Rimonabant | [3H]SR141716A
Type:
Small organic molecule
Emp. Form.:
C22H21Cl3N4O
Mol. Mass.:
463.787
SMILES:
Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1