Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Glucocorticoid receptor
Ligand
BDBM50151068
Substrate
n/a
Meas. Tech.
ChEMBL_302570 (CHEMBL875218)
Ki
0.22±n/a nM
Citation
 von Geldern, TW; Tu, N; Kym, PR; Link, JT; Jae, HS; Lai, C; Apelqvist, T; Rhonnstad, P; Hagberg, L; Koehler, K; Grynfarb, M; Goos-Nilsson, A; Sandberg, J; Osterlund, M; Barkhem, T; Höglund, M; Wang, J; Fung, S; Wilcox, D; Nguyen, P; Jakob, C; Hutchins, C; Färnegårdh, M; Kauppi, B; Ohman, L; Jacobson, PB Liver-selective glucocorticoid antagonists: a novel treatment for type 2 diabetes. J Med Chem 47:4213-30 (2004) [PubMed] Article
von Geldern, TW; Tu, N; Kym, PR; Link, JT; Jae, HS; Lai, C; Apelqvist, T; Rhonnstad, P; Hagberg, L; Koehler, K; Grynfarb, M; Goos-Nilsson, A; Sandberg, J; Osterlund, M; Barkhem, T; Höglund, M; Wang, J; Fung, S; Wilcox, D; Nguyen, P; Jakob, C; Hutchins, C; Färnegårdh, M; Kauppi, B; Ohman, L; Jacobson, PB Liver-selective glucocorticoid antagonists: a novel treatment for type 2 diabetes. J Med Chem 47:4213-30 (2004) [PubMed] Article More Info.:
Target
Name:
Glucocorticoid receptor
Synonyms:
GCR_HUMAN | GR | GRL | Glucocorticoid | Glucocorticoid receptor (GRFP) | NR3C1 | Nuclear receptor subfamily 3 group C member 1
Type:
Enzyme
Mol. Mass.:
85656.87
Organism:
Homo sapiens (Human)
Description:
P04150
Residue:
777
Sequence:
MDSKESLTPGREENPSSVLAQERGDVMDFYKTLRGGATVKVSASSPSLAVASQSDSKQRRLLVDFPKGSVSNAQQPDLSKAVSLSMGLYMGETETKVMGNDLGFPQQGQISLSSGETDLKLLEESIANLNRSTSVPENPKSSASTAVSAAPTEKEFPKTHSDVSSEQQHLKGQTGTNGGNVKLYTTDQSTFDILQDLEFSSGSPGKETNESPWRSDLLIDENCLLSPLAGEDDSFLLEGNSNEDCKPLILPDTKPKIKDNGDLVLSSPSNVTLPQVKTEKEDFIELCTPGVIKQEKLGTVYCQASFPGANIIGNKMSAISVHGVSTSGGQMYHYDMNTASLSQQQDQKPIFNVIPPIPVGSENWNRCQGSGDDNLTSLGTLNFPGRTVFSNGYSSPSMRPDVSSPPSSSSTATTGPPPKLCLVCSDEASGCHYGVLTCGSCKVFFKRAVEGQHNYLCAGRNDCIIDKIRRKNCPACRYRKCLQAGMNLEARKTKKKIKGIQQATTGVSQETSENPGNKTIVPATLPQLTPTLVSLLEVIEPEVLYAGYDSSVPDSTWRIMTTLNMLGGRQVIAAVKWAKAIPGFRNLHLDDQMTLLQYSWMFLMAFALGWRSYRQSSANLLCFAPDLIINEQRMTLPCMYDQCKHMLYVSSELHRLQVSYEEYLCMKTLLLLSSVPKDGLKSQELFDEIRMTYIKELGKAIVKREGNSSQNWQRFYQLTKLLDSMHEVVENLLNYCFQTFLDKTMSIEFPEMLAEIITNQIPKYSNGNIKKLLFHQK
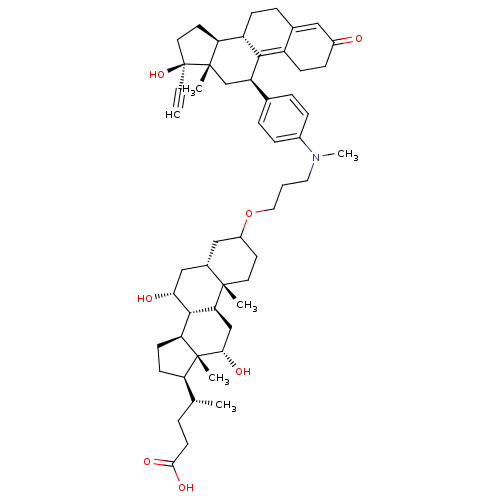
Inhibitor
Name:
BDBM50151068
Synonyms:
(4R)-4-[(1S,2S,7R,9R,10R,11S,14R,15R,16S)-5-[3-({4-[(10S,11S,14R,15S,17R)-14-ethynyl-14-hydroxy-15-methyl-5-oxotetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-1,6-dien-17-yl]phenyl}(methyl)amino)propoxy]-9,16-dihydroxy-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadecan-14-yl]pentanoic acid | CHEMBL415621
Type:
Small organic molecule
Emp. Form.:
C54H75NO7
Mol. Mass.:
850.1758
SMILES:
C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4CC(CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C)OCCCN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#C)[C@@H]2CCC3=CC(=O)CCC3=C12 |t:60,67|