Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Multidrug and toxin extrusion protein 1
Ligand
BDBM50228080
Substrate
n/a
Meas. Tech.
ChEMBL_934320 (CHEMBL2320306)
IC50
18600±n/a nM
Citation
 Wittwer, MB; Zur, AA; Khuri, N; Kido, Y; Kosaka, A; Zhang, X; Morrissey, KM; Sali, A; Huang, Y; Giacomini, KM Discovery of potent, selective multidrug and toxin extrusion transporter 1 (MATE1, SLC47A1) inhibitors through prescription drug profiling and computational modeling. J Med Chem 56:781-95 (2013) [PubMed] Article
Wittwer, MB; Zur, AA; Khuri, N; Kido, Y; Kosaka, A; Zhang, X; Morrissey, KM; Sali, A; Huang, Y; Giacomini, KM Discovery of potent, selective multidrug and toxin extrusion transporter 1 (MATE1, SLC47A1) inhibitors through prescription drug profiling and computational modeling. J Med Chem 56:781-95 (2013) [PubMed] Article More Info.:
Target
Name:
Multidrug and toxin extrusion protein 1
Synonyms:
MATE-1 | MATE1 | S47A1_HUMAN | SLC47A1 | Solute carrier family 47 member 1 | hMATE-1
Type:
PROTEIN
Mol. Mass.:
61928.01
Organism:
Homo sapiens (Human)
Description:
ChEMBL_934313
Residue:
570
Sequence:
MEAPEEPAPVRGGPEATLEVRGSRCLRLSAFREELRALLVLAGPAFLVQLMVFLISFISSVFCGHLGKLELDAVTLAIAVINVTGVSVGFGLSSACDTLISQTYGSQNLKHVGVILQRSALVLLLCCFPCWALFLNTQHILLLFRQDPDVSRLTQTYVTIFIPALPATFLYMLQVKYLLNQGIVLPQIVTGVAANLVNALANYLFLHQLHLGVIGSALANLISQYTLALLLFLYILGKKLHQATWGGWSLECLQDWASFLRLAIPSMLMLCMEWWAYEVGSFLSGILGMVELGAQSIVYELAIIVYMVPAGFSVAASVRVGNALGAGDMEQARKSSTVSLLITVLFAVAFSVLLLSCKDHVGYIFTTDRDIINLVAQVVPIYAVSHLFEALACTSGGVLRGSGNQKVGAIVNTIGYYVVGLPIGIALMFATTLGVMGLWSGIIICTVFQAVCFLGFIIQLNWKKACQQAQVHANLKVNNVPRSGNSALPQDPLHPGCPENLEGILTNDVGKTGEPQSDQQMRQEEPLPEHPQDGAKLSRKQLVLRRGLLLLGVFLILLVGILVRFYVRIQ
Inhibitor
Name:
BDBM50228080
Synonyms:
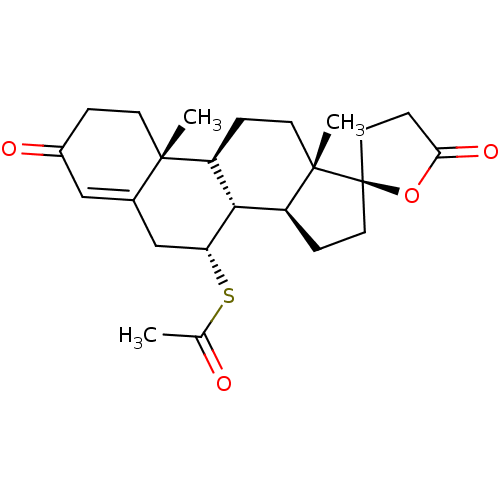
2',15'-dimethyl-5,5'-dioxo-(9'R)-spiro[tetrahydrofuran-2,14'-tetracyclo[8.7.0.02,7.011,15]heptadec-6'-ene]-9-yl ethanethioate | 2',15'-dimethyl-5,5'-dioxospiro[tetrahydrofuran-2,14'-tetracyclo[8.7.0.02,7.011,15]heptadec-6'-ene]-9-yl ethanethioate | 2',15'-dimethyl-5,5'-dioxospiro[tetrahydrofuran-2,14'-tetracyclo[8.7.0.02,7.011,15]heptadec-6'-ene]-9-yl ethanethioate(Spiranolactone) | Aldactone | CHEMBL1393 | S-(2'R,7R,8R,9S,10R,13S,14S)-10,13-dimethyl-3,5'-dioxo-1,2,3,4',5',6,7,8,9,10,11,12,13,14,15,16-hexadecahydro-3'H-spiro[cyclopenta[a]phenanthrene-17,2'-furan]-7-yl ethanethioate | SPIRONOLACTONE | Spiranolactone | cid_5833
Type:
Small organic molecule
Emp. Form.:
C24H32O4S
Mol. Mass.:
416.573
SMILES:
CC(=O)S[C@@H]1CC2=CC(=O)CC[C@]2(C)[C@H]2CC[C@@]3(C)[C@@H](CC[C@@]33CCC(=O)O3)[C@H]12 |r,t:6|